Oil Drop Experiment
Who Did the Oil Drop Experiment?
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron. Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general.
What is the Oil Drop Experiment?
Apparatus
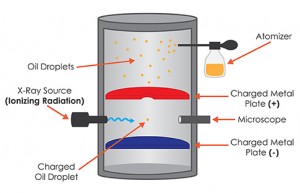
In the original version, Millikan and one of his graduate students, Harvey Fletcher, took a pair of parallel horizontal metallic plates. A uniform electric field was created in the intermediate space by applying a potential difference between them. The plates were held apart by a ring of insulating material. The ring had four holes, three for allowing light to illuminate the setup, and the fourth one enabled a microscope for viewing. A closed chamber with transparent walls was fitted above the plates.
At the beginning of the experiment, a fine mist of oil droplets was sprayed into the chamber. In modern setups, an atomizer replaces the oil droplets. The oil was so chosen such that it had a low vapor pressure and capable of charging. Some of the oil drops became electrically charged by friction as they forced their way out of the nozzle. Alternatively, charging could also be induced by incorporating a source of ionizing radiation, such as an X-Ray tube, in the apparatus. The droplets entered the space between the plates and raised or fell, according to the requirement, by varying plate voltage.
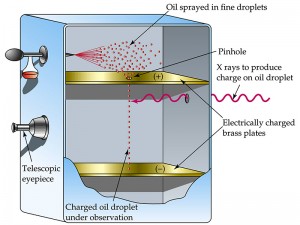
Method
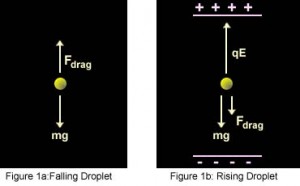
In terms of the present-day arrangement, when the electric field is turned off, the oil drops fall between the plates under the action of gravity only. The friction with the oil molecules in the chamber makes them reach their terminal velocity fast. The terminal velocity is the constant speed that a freely falling object eventually reaches when the resistance of the medium through which it is falling prevents further acceleration. Once the field is turned on, the charged drops start to rise. This motion happens since the electric force directed upwards is stronger than the gravitational force acting downwards. One charged drop is selected and kept at the center of the field of view of the microscope after allowing all other drops to fall by alternately switching off the voltage source. The experiment is conducted with this drop.
Theory and Calculations
First, the oil drop is allowed to fall in the absence of an electric field, and its terminal velocity, say v1, is found out. Using Stokes’ law, the drag force acting on the drop is calculated using the following formula.
Here r is the radius of the drop and ɳ, the viscosity of air.
The weight of the drop, w’, which is the product of its mass and acceleration due to gravity g, is given by the equation,
where ρ is the density of the oil.
However, what we need here is the apparent weight w of the drop in the air given by the difference of the actual weight and the upthrust of the air. We can express w by the following formula.
——-(i)
Here ρair denotes the density of air.
When the drop attains terminal velocity, then it has no acceleration. Hence, the total force acting on it must be zero. That means,
or,
or,
The above equation can be used to find out the value of r. Once r is calculated, the value of w can easily be found out from equation (i) marked above.
Now after turning on the electric field between the plates, the electric force FE acting on the drop is,
Where E is the electric field and q the charge on the oil drop. For parallel plates, the formula for E is,
Here V is the potential difference and d the distance between the plates. That implies,
Now if we adjust V to make the oil drop remain steady at a point, then
or,
or,
Thus, the value of q can be calculated. By repeatedly applying this method to multiple oil droplets, the electric charge values on individual drops were always found to be integer multiples of the smallest value. This lowest charge could be nothing but the charge on the elementary particle, electron. By this method, the electronic charge was calculated to be approximate, 1.5924×10−19 C, making an error of 1% of the currently accepted value, 1.602176487×10−19 C. All subsequent research pointed to the same value of charge on the fundamental particle.
Conclusion
Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself. Millikan’s oil drop experiment proved that the electric charge is quantized in nature. The electric charge appears in quanta of magnitude 1.6 X 10-19 C in oil droplets.
Robert Millikan’s Oil Drop Experiment Animation
Millikan’s Oil Drop Experiment and the Atomic Theory
Until the time of the Oil Drop Experiment, the world had little or no knowledge of what is present inside an atom. Earlier experiments by the English Physicist J.J. Thomson had shown that atoms contain some negatively charged particles of masses significantly smaller than that of the hydrogen atom. Nevertheless, the exact value of the charge carried by these subatomic particles remained in the dark. The very existence of these particles was not accepted by many due to a lack of concrete evidence. Thus, the atomic model was shrouded in mystery. In this scenario, with Millikan’s groundbreaking effort to quantify the charge on an electron, the atomic theory came of age in the early years of the twentieth century.
Controversy about the Oil Drop Experiment and Discovery
Robert Millikan was the sole recipient of the Nobel Prize in Physics in 1923 for both his work in this classic experiment and his research in the photoelectric effect. Fletcher’s work on the oil drop project, however, was not recognized. Many years later, the writings of Fletcher revealed that Millikan wished to take the sole credit for the discovery in exchange for granting him a Ph.D. and helping him secure a job after his graduation.
The beauty of the oil drop experiment lies in its simple and elegant demonstration of the quantization of charge along with measuring the elementary charge on an electron that finds widespread applications to this day. With the progress of time, considerable modifications have been made to the original setup resulting in obvious perfection in the results. Still, no substantial deviation from the results of the classical experiment could yet be found.
Summary
- Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron. The experiment was the first direct and riveting measurement of the electric charge of a single electron.
- They suspended tiny charged droplets of oil between two metal electrodes by balancing downward gravitational force with upward drag and electric forces.
- They later used their findings to determine the mass of the electron.
-
References
Article was last reviewed on Thursday, February 2, 2023