Dephosphorylation
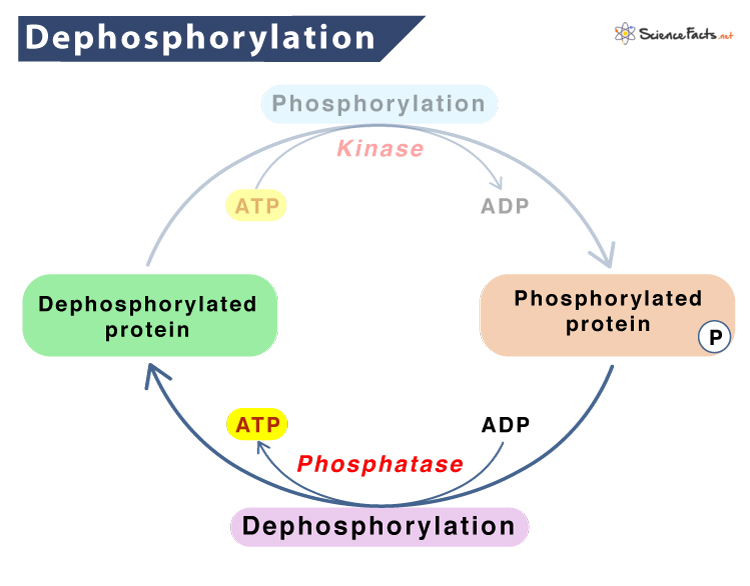
Dephosphorylation is the process in which phosphate groups (PO43−) are removed from an organic molecule, such as a protein, by a hydrolytic enzyme or hydrolase. It is thus the opposite of phosphorylation, where phosphate is added. Along with phosphorylation, it helps to activate or deactivate enzymes that cleave the ester bond by hydrolysis.
A typical example of hydrolase is a phosphatase, which removes phosphate groups by hydrolyzing phosphoric acid monoesters forming a phosphate ion and a molecule having a free hydroxyl group.
Edwin Krebs and Edmond Fischer discovered dephosphorylation as a reversible process in functional proteins that are mainly enzymatic and non-enzymatic.
Mechanism of Dephosphorylation
Dephosphorylation involves the removal of the phosphate group by a hydration reaction, adding a molecule of water, and releasing the original phosphate group, regenerating the hydroxyl group used up in phosphorylation.
Functions of Dephosphorylation
The reversible phosphorylation-dephosphorylation reaction of hydroxyl groups in neutral, polar amino acids such as serine, threonine, and tyrosine in target proteins regulates all physiologic processes within the cell.
Like phosphorylation, dephosphorylation occurs on all substrates, such as structural proteins, enzymes, membrane channels, and signaling molecules. These processes are called phosphoregulation.
Some common examples of dephosphorylation occurring in living systems are:
1. Post-translational Modifications
Proteins synthesized by translation need to be processed to form a mature protein. After the modifications, a protein can assume its function(s) in a cell.
2. Dephosphorylation of ATP
As part of post-translational modifications, ATP’s dephosphorylation involves removing phosphate groups from amino acids serine, threonine, or tyrosine.
ATP4− + H2O ⟶ ADP3− + HPO42− + H+
In the spontaneous conversion of ATP to ADP by ATPase, 30.5 kJ/mol of energy is released, which drives various cellular reactions. Even non-spontaneous reactions such as oxidative phosphorylation become spontaneous when coupled to the dephosphorylation of ATP.
Components of protein synthesis also undergo phosphorylation and dephosphorylation and thus regulate the rate of protein synthesis.
At the cellular level, the dephosphorylation of ATPases determines the relative flow of ions in and out. Proton-pump inhibitors function by disrupting the ATPases.
3. Dephosphorylation in Other Reactions
Some other organic molecules that undergo dephosphorylation in a cell are glucose-6-phosphate, phosphoenolpyruvate, acetyl phosphate, and phosphocreatine. They release different amounts of energy that the cell uses to perform other cellular reactions.
4. Activating Photosystem II
Protein dephosphorylation in photosynthetic thylakoid membranes at high temperatures also stimulates the dephosphorylation of some proteins in the photosystem II complex and thus activating its function during photosynthesis.
5. Role in Diseases
- Excessive dephosphorylation of the membrane-bound ATPases and proton pumps in the gastrointestinal tract stimulates the synthesis of caustic peptic acids. Such a condition results in heartburn and esophagitis. Along with Helicobacter pylori infection, it can cause peptic ulcer disease.
- In patients having Alzheimer’s disease, the microtubule-associated protein tau is found to be hyperphosphorylated. Such condition results due to the abnormality in enzymes protein phosphatase-2A or phosphatase-2B or both at specific amino acids on the tau protein.
- Dephosphorylation alters the actin-myosin interactions and thus is linked to cardiac diseases. When the dephosphorylation process is interrupted, calcium-dependent cardiac contraction is also impaired or fully disabled.
- It is also found to affect the insulin signaling pathway through dephosphorylation of the insulin receptor substrate-1/2, Akt, and ERK1/2, phosphoproteins, and thus has implications in type II diabetes.
6. Molecular Research
Dephosphorylation plays an essential role in cloning involving restriction enzymes. For ligation to happen, the cut end of a vector must not re-ligate after restriction digestion. It is achieved by the enzyme alkaline phosphatase isolated from the calf intestine (abbreviated as CIP), which removes the phosphate group present at the 5′ termini of a DNA molecule.
FAQs
Ans. The main difference is that a phosphate group is added to a molecule by the enzyme protein kinase during phosphorylation. In contrast, dephosphorylation involves the removal of a molecule’s phosphate group by the enzyme hydrolase, especially by a phosphatase.
-
References
Article was last reviewed on Wednesday, January 4, 2023