Fermentation
Fermentation is a biochemical process in which carbohydrates like glucose or starch are converted to alcohol or acid without oxygen. Microorganisms like yeasts, anaerobic bacteria, and muscle cells in animals use fermentation as a means of producing ATP without the presence of oxygen. Thus, fermentation is a form of anaerobic respiration.
It involves glycolysis but not the other two stages of aerobic respiration. Like glycolysis, fermentation occurs in the cytoplasm of both prokaryotic and eukaryotic cells.
‘Ferment’ comes from the Latin word fervere, meaning ‘to boil’. The science of fermentation is called zymology. Some common fermented food beverages are wine, beer, cheese, yogurt, sauerkraut, kimchi, and pepperoni.
A Short History: Timeline
- Fermentation dates back to 10,000 BCE when early humans used to preserve milk from cattle, sheep, goats, and camels.
- The scientific investigation began in the 16th century. People used fermentation to make products such as wine, cheese, and beer long before the process was correctly understood.
- In the 1850s and 1860s, Louis Pasteur described how fermentation was caused in living cells. However, he could not extract the enzyme necessary for fermentation in yeasts.
- In 1897, German chemist Eduard Buechner extracted the fluid responsible for fermentation, which earned him Nobel Prize in 1907.
Why Does Fermentation Occur in the Cell
Fermentation and cellular respiration begin the same way, using glycolysis. However, due to a lack of oxygen, pyruvate formed in glycolysis does not enter the Kreb’s cycle and oxidative phosphorylation through the electron transport chain (ETC).
The ETC being non-functional, the NADH made in glycolysis cannot transfer its electrons and revert to NAD+. However, the NADH must be oxidized to allow cells to continue making ATPs using glycolysis. In this condition, fermentation is necessary for the cell.
How does Fermentation Allow Glycolysis to Continue
In fermentation, pyruvate produced through glycolysis is reduced, and NADH is oxidized to NAD+. Regeneration and a steady supply of NAD+ allow glycolysis to continue, thus providing the cell with the energy necessary for all metabolic functions.
How Many ATPs are produced in Fermentation
Cells can make only 2 ATPs at most per fermentation as oxidative phosphorylation remains non-functional.
How does Fermentation Work, and What are its Types
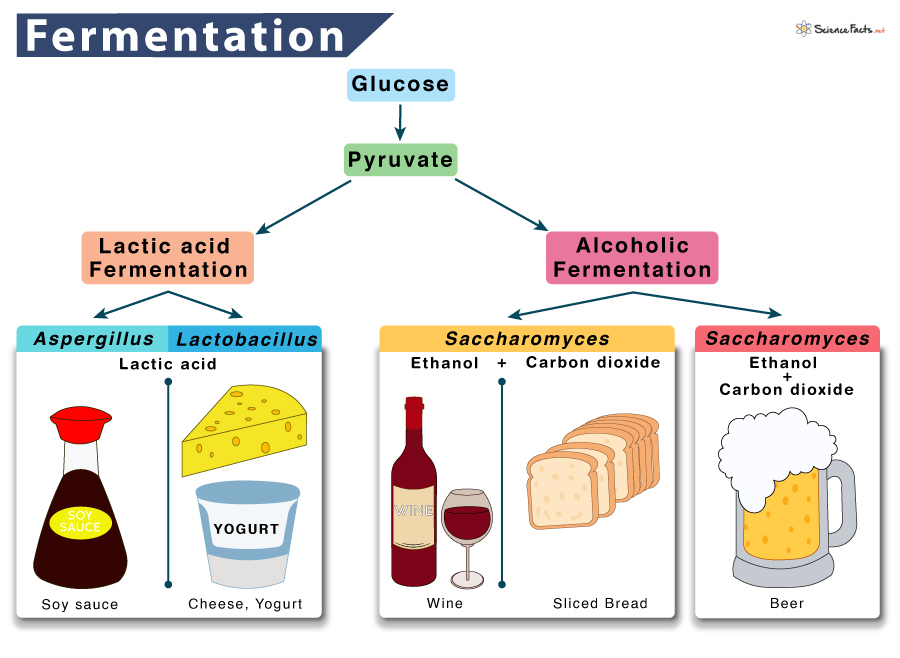
Fermentation is a two-step reaction. The exact step depends on the type of fermentation under study. The two main types of fermentation found in living organisms are 1) lactic acid fermentation and 2) alcoholic fermentation.
1. Lactic Acid Fermentation
The pyruvate produced through glycolysis is converted to lactate or lactic acid by the fungus Aspergillus. The enzyme that catalyzes this step is lactate dehydrogenase, lactic acid being the byproduct.
Humans carry out lactic acid fermentation when the body needs much energy, such as during sprinting and workouts. Once the stored ATP in the cell is used up, our muscles start producing ATP through lactic acid fermentation. Lactic acid fermentation also occurs in the bacteria Lactobacillus,which is used in making cheese and yogurt.
Equation
Glucose → 2 Pyruvate + 2 NADH → 2 Lactic Acid + 2 NAD+
2. Alcoholic Fermentation
When yeasts such as Saccharomyces are kept without oxygen, they switch to alcoholic fermentation to generate usable energy from food. Similar to lactic acid and ethyl alcohol, the product of alcoholic fermentation is also a byproduct. It is a two-step process.
Step 1: A carboxyl group is removed from pyruvate and released as carbon dioxide, producing a two-carbon molecule called acetaldehyde.
Step 2: NADH passes its electrons to acetaldehyde, regenerating NAD+ and ethyl alcohol.
It is the process that causes bread dough to rise. When yeast cells in the dough run out of oxygen, the dough begins to ferment, producing tiny bubbles of carbon dioxide as their waste products. These bubbles are the air spaces we see in a slice of bread. Alcoholic fermentation is also used in wine production.
Equation
Glucose → 2 Pyruvate + 2 NADH →2 Ethanol + 2 NAD++ 2CO2
What is the Purpose of Fermentation
Main Function
- To regenerate NAD+ by oxidizing NADH, the electron carriers in glycolysis. Thus, fermentation ensures an uninterrupted supply of ATP to the cell.
Commercial Applications
Fermentation also has high commercial importance.
- To produce fermented food and beverages. For example, wine, beer, cheese, yogurt, sauerkraut, kimchi, and pepperoni are high in nutritional value and can act as probiotics.
- To produce methane and hydrogen gas
- To neutralize anti-nutrients like phytic acid in grains, nuts, seeds, and legumes, thus increasing their nutritional value
Anaerobic Cellular Respiration
Some bacteria and archaea (methanogens) found in the soil and the digestive tracts of cows and sheep do not perform fermentation. However, they use an alternative way to regenerate NAD+. They reduce carbon dioxide to methane to oxidize NADH.
Likewise, sulfate-reducing bacteria and archaea reduce sulfate to hydrogen sulfide to regenerate NAD+ from NADH.
FAQs
Ans. Fermentation is less effective than aerobic respiration because it cannot wholly break down glucose molecules. Unlike aerobic respiration, it can produce only two ATPs via glycolysis, compared to 36 ATPs in aerobic respiration.
Ans. Fermentation is a catabolic reaction.
Ans. The main difference between glycolysis and fermentation is that glycolysis can occur in the presence or absence of oxygen. In contrast, fermentation strictly occurs in the absence of oxygen.
Ans. Lactic acid and alcoholic fermentation both occur in the absence of oxygen.
Ans. The final electron acceptor in fermentation is an organic compound.
-
References
Article was last reviewed on Tuesday, August 23, 2022