Phagocytosis
What is Phagocytosis
Phagocytosis combines two Greek words, where ‘phagein’ means ‘to eat’ and ‘kytos’ denotes ‘cell’.
It is a class of endocytosis where specific living cells ingest or engulf other cells or particles of size greater than 0.5 μm, forming a vesicular structure. The cell that performs phagocytosis is called a phagocyte, and the vesicular structure is called a phagosome.
Canadian physician William Osler first observed this phenomenon in 1876. It was later studied and named by Russian microbiologist Élie Metchnikoff (1880, 1883). In 1908, he received Nobel Prize for the same.
Phagocytosis differs from other methods of endocytosis due to its specificity. It depends on the ability of the cell to bind to the target through surface receptors. Both the cell and the foreign particle must be in physical contact for this process to occur.
What Cells Perform Phagocytosis
Most of the cells are capable of phagocytosis. However, several types of immune cells have specialization in this role.
Depending on their efficiency, the phagocytes are of two types.
a. Professional Phagocytes: They perform phagocytosis as their primary function. They have receptors on their cell surface, which help them to detect any pathogen. So, their primary role is to locate and engulf invading foreign organisms like bacteria and clear dead cells, thus protecting the body. White blood cells (WBCs), including monocytes, macrophages, neutrophils, dendritic cells, and mast cells, fall under this category.
b. Non-Professional Phagocytes: Cells like epithelial cells, endothelial cells, mesenchymal cells, and fibroblasts are classified as non-professional phagocytes as their principal function is not phagocytosis. For example, fibroblasts, which can phagocytose collagen in the process of remolding scars, can also make some attempts to ingest foreign particles. These types of cells lack efficient phagocytic receptors.
Does It Require Energy
Phagocytosis is a type of active transport mechanism as it requires the direct use of ATP to fuel the transport.
Where and When Does It Occur
Phagocytosis occurs in almost any tissue, most often in the bloodstream and interstitial space.
Major organs like lungs, liver, spleen, and lymph nodes, rich in macrophages, perform phagocytosis – for example, the Kupffer cells in the liver.
It is principally a defensive reaction against infection and invaded foreign substances (antigens). Therefore, it is used by the innate immune system when an infected cell is trying to destroy a foreign particle, like a virus. However, in a free-living single-celled phagocyte, such as an amoeba, this process is used as a means of feeding.
How does Phagocytosis Work
Phagocytosis is a complex phenomenon involving a cascade of multiple molecular mechanisms. So, the cells need to undergo specific steps in order to phagocytize something.
Steps
There are eight stages or steps involved in phagocytosis. They are described below:
Step 1: Activation of the Phagocyte
It is the first step of phagocytosis when the resting phagocytes become activated. During this phase, the phagocytes come close to some inflammatory mediators like bacterial proteins, capsules, peptidoglycan, prostaglandins, and complement proteins. As a result, the receptors on the cell surface bind to these molecules and cause the cells to respond.
Consequently, the phagocytes switch to a higher energy level. This phenomenon usually involves rearranging the cell cytoskeleton and swelling of the cell (caused by calcium and sodium ion influx).
Phagocytes also produce pattern recognition receptors (PRRs) which recognize and bind to pathogen-associated molecular patterns (PAMPs). PAMPs are components of pathogens and can include molecules like peptidoglycan and lipopolysaccharide (LPS).
Step 2: Chemotaxis of the Phagocyte
In the next step, the directional movement of the phagocyte occurs toward a higher concentration of molecules under the influence of a chemical attractant called chemotaxin. Bacterial products (e.g., endotoxin), injured tissues, complement proteins, and chemical substances produced by WBCs are typical examples of chemotaxin.
Activated cells express more glycoprotein receptors that help them reach the site of infections and bind firmly with microorganisms under the influence of cytokines.
Step 3: Attachment of the Phagocyte to the Pathogen
Here, receptors present on the cell surface of the phagocyte bind or adhere to the surface of the pathogen. This step is necessary for the ingestion of foreign particles. However, some bacteria can resist attachment, making it harder for the phagocytic cell to be taken into the cell and destroyed.
Different types of cells express different types of receptors; some are general, while some are specific. However, depending on the cell, there are mainly four types of surface receptors that play an essential role in phagocytosis (binding). They are given below:
Opsonin Receptors: These general transmembrane receptors are present on the cell surface of phagocytes (macrophages and neutrophils) and act via specific antimicrobial proteins called opsonins. In Greek, ‘opson’ means to ‘prepare for eating.’
These molecules enhance phagocytosis efficiency by phagocytic cells either by activating the complement pathway or marking the antigen with a specific antibody which makes it easier to recognize by the respective receptor. The process of coating pathogens to promote phagocytosis is called opsonization.
Scavenger Receptors: Scavenger receptors are a general type of Pattern recognition receptors (PRRs). Thus they recognize pathogen-associated molecular patterns (PAMPs) such as peptidoglycan, teichoic acids, lipopolysaccharide, mannans, flagellin, pilin, and bacterial DNA.
Macrophages widely express scavenger receptors (SRs). These receptors can bind to a diverse array of endogenous and non-self or foreign molecules.
Toll-like Receptors (TLRs): These receptors get their name from a similar receptor found in fruit flies (Drosophila melanogaster) encoded by the Toll gene. They are also a type of PRR and bind to specific molecules produced by bacteria, which activates the immune response.
TLRs have numerous roles. For example, they help recognize self and non-self antigens, detect invading pathogens, bridge innate and adaptive immunity, and regulate cytokine production, proliferation, and survival.
Antibodies: Some immune cells produce antibodies that can identify and bind to specific antigens, thus neutralizing or destroying the foreign object. Like Toll-like receptors, antibodies are also particular in their action, i.e., a particular antibody only act against a definite antigen. This feature is called antibody specificity.
Antibodies stimulate phagocytosis by coating the pathogen, making it more accessible to the phagocytes, thus playing a significant role in opsonization. Also, antibodies like IgM trigger the destruction of pathogens by stimulating the complement pathway.
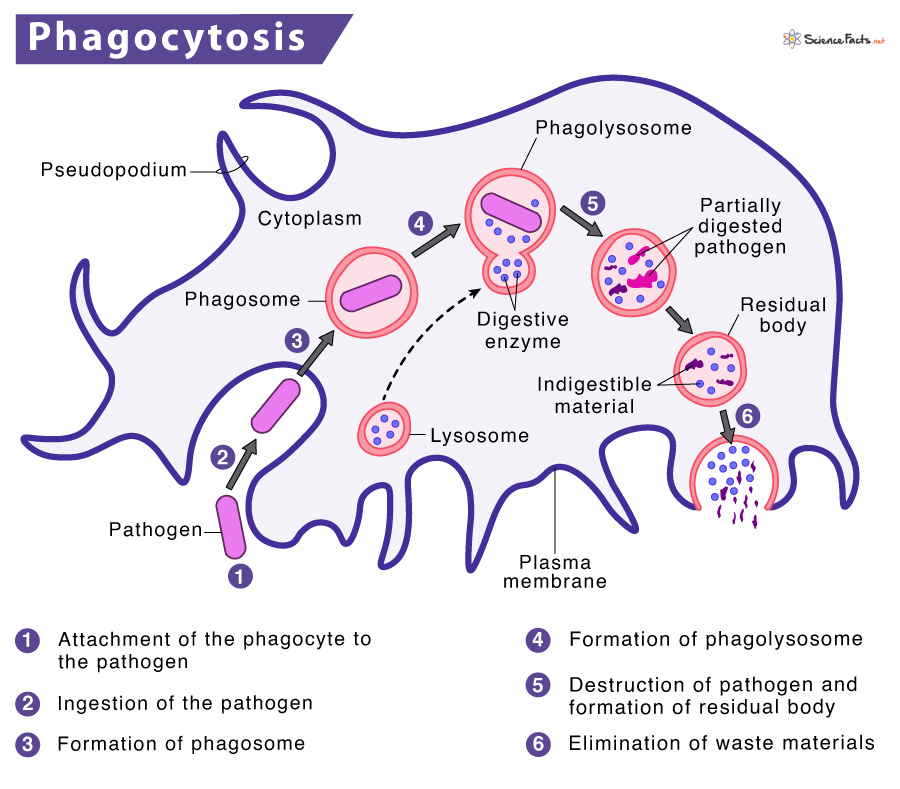
Step 4: Ingestion of the Pathogen
After the attachment of the phagocyte to the pathogen, the cell begins to engulf the foreign particle. Then, the phagocyte starts extending the cytoplasm (pseudopods) as it surrounds the molecule to avoid the risk of membrane damage. As the cells are reasonably flexible and fluid, the pseudopods protrude outward on either side of the particle until both ends meet.
Step 5: Formation of Phagosome
When a pathogen or opsonin binds to a cell receptor, it triggers actin filaments within the cell. As a result, the actins get polymerized and form pseudopodia after the ingestion of the pathogen, which then surrounds the engulfed microorganism. These two protruding arms fuse, forming a vesicular structure called a phagosome. Finally, the phagosome transports the particle into the cell.
Step 6: Formation of Phagolysosome
Upon entering the cell, the phagosome fuses with a lysosome, becoming a phagolysosome. Lysosomes contain hydrolytic enzymes that help in the digestion of phagocytized particles. However, for phagocytes involved in immunity, unique structures called peroxisomes are produced to trap and remove toxic molecules.
Step 7: Destruction of the Pathogen
Phagolysosomes reduce the pH of their internal environment, making them acidic. This activity serves as an effective defense mechanism against microbes and provides a suitable medium for degradative enzyme activity.
As lysosomes contain the digestive enzyme called lysozyme and various antimicrobial and cytotoxic substances, they destroy the phagocytosed pathogen. The microbes are then killed either by oxygen-dependent (oxidative) or by oxygen-independent (non-oxidative) mechanisms.
Some other ways of destroying pathogens are using oxygen radicals, nitric oxide, antimicrobial proteins and peptides, and binding proteins.
Step 8: Elimination of Waste Materials
This is the final step of phagocytosis. Once the digested contents of the phagolysosome get neutralized, it forms a residual body containing the waste products. The wastes are then discharged from the cell by exocytosis, thus completing the process.
What Functions Does it Serve with Examples
Main Function
As discussed above, phagocytosis helps to protect us from the invasion of foreign particles such as bacteria, viruses, and protozoa and thus plays a vital role in the immune response.
Role in Apoptosis
Phagocytic cells remove apoptotic cells (dead cells) by efferocytosis. This process is known as the burying of dead cells. Both professional and non-professional cells can ingest dead cells. It plays a significant role in resolving inflammation and protecting tissue from harmful exposure to dying cells, inflammatory and immunogenic contents. It also contributes to tissue homeostasis.
FAQs
Ans. Helper T cells increase phagocytosis and antibody formation.
Ans. Autophagy is a process by which a cell removes its unnecessary or dysfunctional components in a regulated manner. It is performed by all cells. In contrast, phagocytosis is a cell’s defense mechanism that destroys a foreign particle like a virus or an infected cell. It is performed by immune system cells.
Ans. Chemotaxis is the movement of organisms or cells towards or away from a chemical stimulus. On the other hand, phagocytosis is a mechanism used by immune cells and certain organisms to engulf infectious particles and destroy them.
-
References
Article was last reviewed on Thursday, February 2, 2023