Acid Rain
What is Acid Rain?
Acid rain, also called acid deposition, is a broad term for any form of precipitation with high concentrations of sulfuric and nitric acids. According to the Environmental Protection Agency (EPA), deposition can be wet such as in rain, fog, snow, mist, or dry, as in dust, gas, and smoke.
The term ‘acid rain’ was used for the first time by Robert Angus Smith in 1852 while examining rainwater reaction from the U.K. and Scotland’s industrial sites.
Where does Acid Rain Occur?
First identified in 1872 in Sweden, scientists in the U.S. started examining the phenomenon in the 1950s. Later in the 1960s and early 1970s, acid rain was recognized as a regional issue that mostly affected Western Europe and North-Eastern America. It is also increasingly found in parts of India and China. Sulfuric acidic rain commonly occurs in Venus.
What Causes Acid Rain and How is it Formed
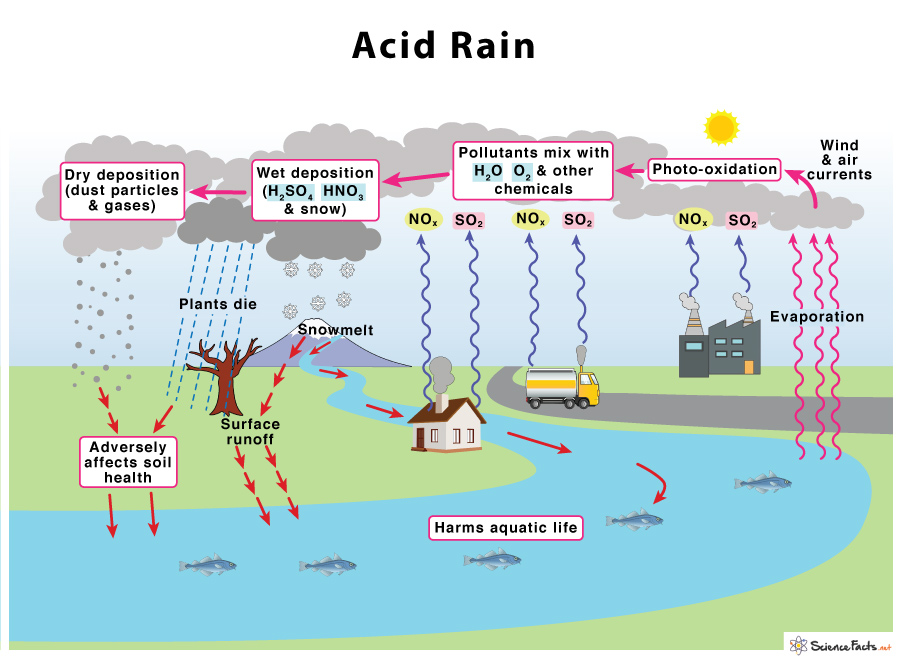
Acid rain forms when two significant pollutants, sulfur dioxide (SO2) and nitrogen oxides (NOX), are released into the atmosphere due to various natural and human activities. While natural sources such as volcanoes, lightning, or decomposing vegetation emit SO2 and NOX, acid rain’s primary cause is human-made. The emission of coal, petroleum, and natural gas from the factories, smelting of ore, and automobile emission are all manmade sources.
The process starts when SO2 and NOX are emitted into the atmosphere, undergo photooxidation in the presence of sunlight and oxygen, and react with water and other components present in the atmosphere. Wind and air currents spread these acidic substances over long distances. They eventually precipitate down in the form of wet deposits such as rain (sulfuric and nitric acids), fog, snow, and mist, or dry deposits as dust, gas, and smoke, adversely affecting soil heath and aquatic life.
How Acidic is Acid Rain?
Normal rain is slightly acidic, having a pH of 5.6. It forms when carbon dioxide reacts with water to form weak carbonic acid, which has no harmful effects.
However, acid rain generally has a pH between 4.2 and 4.4. Here, sulfur dioxide and nitrogen dioxide undergo oxidation before reacting with water. The following equations show the formation of acid rain:
Formation of Sulfuric Acid
SO2 (g) + H2O (l) → H2SO3 (aq)
H2SO3 (aq) + O2 (g) → 2H2SO4 (aq)
Formation of Nitric Acid
N2 (g) +O2 (g) → 2NO(g)
2NO (g) + O2 (g) → 2NO2 (g)
2NO2 (g) + H2O (l) → HNO3 (aq) + HNO2 (aq)
Since nitrous acid is unstable, it eventually oxidizes to nitric acid. Thus, the overall reaction is:
2N2 (g) + 5O2 (g) + 2H2O (l) → 4HNO3 (aq)
How does Acid Rain Affect the Environment?
Acid rain nearly affects everything around us. Some significant consequences are listed below:
1) Effects on Soil: It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. It causes a change in soil composition, thus affecting crop production. Acid rain also affects forests, especially those at higher elevations.
2) Effects on Plants: It weakens the trees by washing away the waxy, protective coating on leaves, thus damaging them. Acid rain washes away the essential nutrients and minerals from the soil, causing stunted growth in plants by affecting photosynthesis.
3) Effects on Water Bodies: Acid rain makes lakes, streams, ponds, rivers, and other water bodies more acidic. It adversely affects aquatic life, such as freshwater shrimps, snails, and mussels. Most fish species cannot survive a pH of less than 5. When the pH becomes 4, the lake is dead, which means it becomes devoid of life. Again, the fall of acid rain causes aluminum absorption from the soil, which is carried into the water bodies. The combination of both makes water bodies more toxic for their survival.
Some aquatic species can tolerate acidic pH better than others. Nevertheless, since all ecosystems are interconnected, organisms in one ecosystem depend on the other for their survival. For example, if a fish species disappear, the animals, including the birds that feed on them, will also become extinct.
4) Effects on Building, Monument, and Statue: All structures, especially those made of limestone and sandstone, are mostly affected by the effect of acid rain. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering.
Corrosion in Taj Mahal is a real-life example. The chemical reaction causing their corrosion is given below:
CaCO3 (s) + H2SO4 (aq) → CaSO4 (s) + H2O (l) + CO2 (g)
Another instance is the Statue of Liberty, which is made of copper. It is also getting damaged by the cumulative action of acid rain and oxidation, thus becoming green.
5) Other Impacts: It causes corrosion of water pipes, which results in the leaching of heavy metals such as lead, iron, and copper into drinking water. This effect can adversely affect humans. Dry precipitation is sometimes associated with heart and lung problems such as asthma and bronchitis.
Solutions to Problems of Acid Rain
The only way to reduce the consequence of acid rain is by checking the emission of nitrogen and sulfur oxides, which can be done by:
- Regulating their emission from coal-based and metal extracting industries by filtering the exhaust before releasing into the environment
- Reducing the dependence on non-renewable energy resources such as coal, petroleum, and natural gas. According to the EPA, this can be done by increasing renewable energy consumption from sunlight, wind, and water.
- Using eco-friendly vehicles instead of petrol or diesel-based ones. The use of catalytic converters filters the exhaust gases from the vehicles before releasing them into the environment.
- Planting more trees or afforestation helps to purify the atmosphere by reducing toxic gases.
- Restoring water-body damage by using powdered limestone that neutralizes the water, a process known as liming.
Advantages of Acid Rain
Here are some examples of acid rain.
- Makes holes in the limestone below ground resulting in caves and groundwater storage and providing habitat for some species
- Sulfuric acid rain limits global warming by offsetting methane’s natural production by microbes in wetland areas, thereby limiting climate change.
FAQs
Ans. Acid rain is still a massive problem in many countries. However, its effect has reduced many folds in Europe and North America compared to the 1970s and 1980s. In the U.S., the Clean Air Act of 1990 has helped cut sulfur dioxide emissions by 88 percent and nitrogen dioxide down by 50 percent between 1990 and 2017. Countries like India and China are still trying to implement control over the emission of such gases.
-
References
Article was last reviewed on Friday, February 3, 2023