Why Does Water Expand When It Freezes
Does Water Expand When It Freezes?
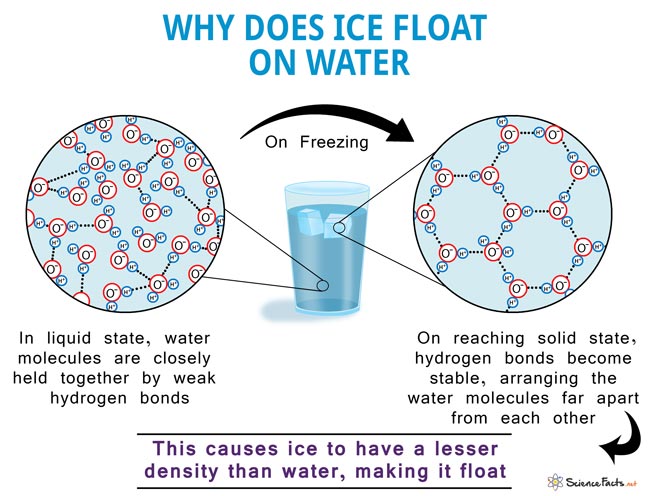
Yes. Ice has a lesser density than water.
How Much Does Water Expand When It Freezes?
Approximately by about 9% – 10%.
Why? – The Cause behind the Effect
On heating, liquids expand since the molecules move with greater energy overcoming the intermolecular attraction. On the contrary, liquids usually contract on cooling. The molecules move slower and cannot overcome the force of attraction between them. When they freeze, they contract more to form a rigid solid structure with minimal intermolecular spaces. But that is not the case with water. Instead of contracting, it expands.
The Molecular Standpoint
The water molecule, consisting of 2 atoms of hydrogen and one of oxygen, forms a Mickey Mouse head-like structure such that the ears are the hydrogen atoms and the oxygen atom represents the head. The oxygen atom side of the molecule is slightly negative, while the hydrogen atoms side has a slightly positive charge. This makes the water molecules drawn towards each other, forming hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Hence, water is said to expand upon freezing and becomes less dense. On the other hand, it contracts on thawing, much unlike most other liquids.
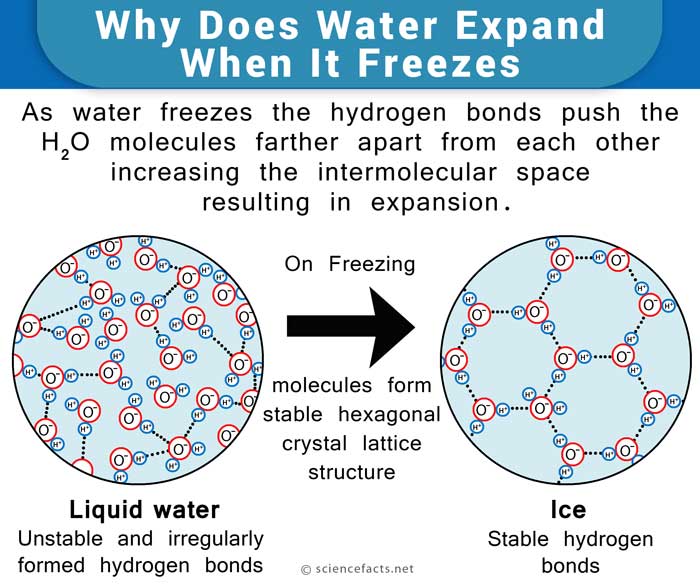
How Does Water Expand When It Freezes?
Water also shows the expected contracting behavior due to cooling up to 4° C. But on cooling to temperatures below that, it starts expanding in volume. This is because the molecules start to get into a stable hexagonal lattice structure, as mentioned above. It is a very open structure with a lot of intermolecular space. Hence the expansion. The same applies when it finally freezes to ice at 0° C.
Is Water The Only Substance That Expands When It Freezes?
No, it is not. Other substances include plutonium, germanium, bismuth, gallium, silicon, etc.
Video
Here is a video explanation to make it simple for you.
This phenomenon, also known as the anomalous expansion of water, is why water bottles crack when water freezes inside. Another effect of this is the floating of ice cubes in water. Ice is of lesser density than water due to the abovementioned reason.
Article was last reviewed on Monday, July 31, 2023






yes, ice has lesser density than water when it freezes? OK………….STOP THE BUS……..its GREATER density when it freezes. It has less vibration and movement. As it happens, it is the vessel allowing expansion. If you freeze water in a fully contained metal box (for example).then when frozen…it will be the same dimensons, other than .05mm – .5mm. The vessels material is the difference. In open air then many variables are of contribution
Thank you so much for this useful article
Just 1 question;
If ice expands when it is frozen, should not water levels decrease if the polar caps thaw.
Well I’m assuming because ice caps aren’t fully submerged levels increase because the ice that was previously only 70% displacing the water is now 100% Incorporated. I’m no scientist tho ????
then water freezes to form ice at 4 0 it expands to become ice. The ice floats to the top. If did not tth oceans would freeze solid making it impossible for life to survive.
“Is Water The Only Substance That Expands When It Freezes?
No, it is not. Other such substances include plutonium, germanium, bismuth, gallium, silicon, acetic acid, etc.”
Hello
I wonder if in fact glacial acetic acid expands when it freezes since its density is 1,049 g cm − 3 (liquid); and 1.27 g cm − 3 (solid)?
Best regards, AC
Thank you for the comment. Acetic acid does not expand when frozen. We have removed it from the text.
it was very explanatory and very good
A very good explanation of a subject that most people don’t understand ????
Can one say that liquid water flows to occupy the minimum space available,but when solid it occupies the exact space
@Barry L Clark:
You may have a point in relation to the _North_ pole ice as it’s all a floating sheet. It’s also not very thick.
South pole / Antarctic ice is another matter. It averages 6500 feet thick and most of it is supported by land, not floating on top of water. I did say *most*, not all. There are “shelves” beyond the land mass which is why some can break off and float away.
@smarter
You’re simply wrong. Water expands when it freezes. Canned soda (or water) will burst the can when it freezes because of this expansion. If it were more dense when it froze, ice cubes would sink to the bottom of a glass of water. If the density stayed the same they would be free to be at the top, bottom or anywhere in between.