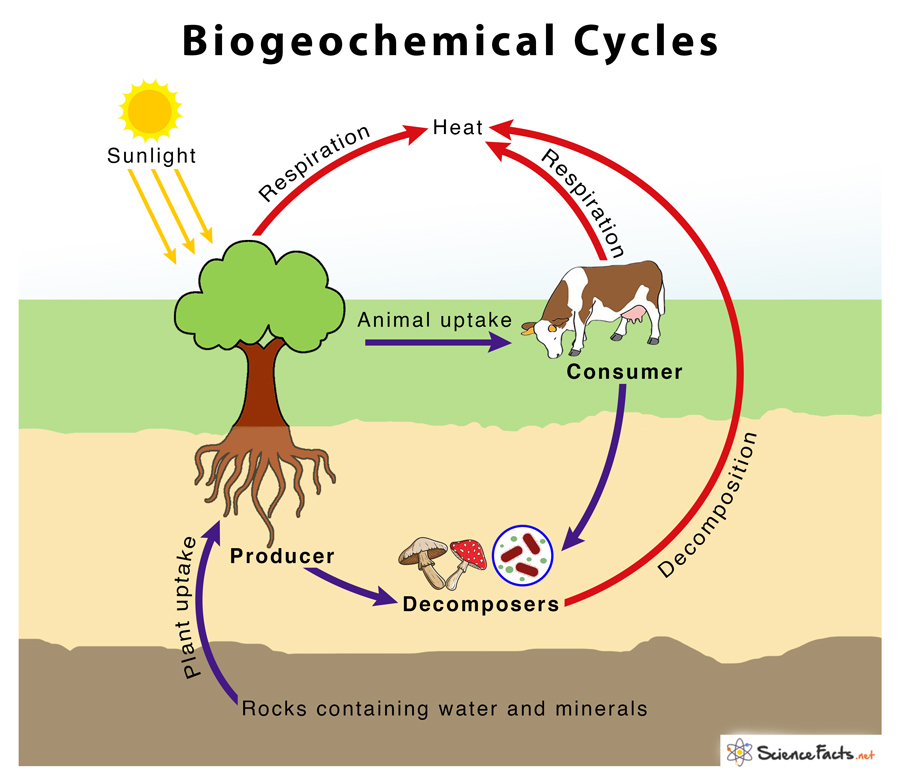
Biogeochemical Cycles
The term biogeochemical is derived from ‘bio’ meaning ‘biosphere’, ‘geo’ meaning ‘the geological components’, and ‘chemical,’ meaning the elements move through a cycle.
Energy flows directionally through the ecosystem, entering as sunlight (for photoautotrophs) or inorganic molecules (for chemoautotrophs) and leaving as heat. The ways in which inorganic elements and some inorganic or organic compounds move between the various living and nonliving forms based on their locations on earth are called biogeochemical cycles.
Types of Biogeochemical Cycles
The two general categories are:
a) Gaseous cycles: Examples include carbon, oxygen, nitrogen, and the water cycle
b) Sedimentary cycles: Examples include sulfur, phosphorous, and rock cycle.
Geologic processes, such as weathering, erosion, infiltration, percolation, and the subduction of the continental plates, play a crucial role in the movement of elements from living to non-living, from the atmosphere to land to water bodies, and from soils to plants on earth.
Among all, the most common biogeochemical cycles are briefly described below.
1. Water Cycle
The water cycle involves changes in the state of water and also the physical movement of water between the different tropic levels.
The hydrosphere is the area of earth involving the atmospheric water in the form of water vapor and clouds, ice in the form of polar ice caps and glaciers, surface water in the form of water bodies such as rivers, lakes, seas, ocean, and the underground water.
Water typically enters the atmosphere as vapors as they evaporate from the water bodies due to the sun’s heat or from the aerial parts of the plants by transpiration. This atmospheric water condenses into droplets to form clouds that return to the water bodies as rain, thus completing the cycle.
2. Carbon Cycle
Autotrophs (produced by green plants and algae) fix atmospheric carbon through photosynthesis to make organic compounds (glucose). Heterotrophs (consumed by humans and other animals) consume autotrophs as the carbon is passed through food chains and food webs.
Carbon is returned into the atmosphere when autotrophs and heterotrophs perform cellular respiration or when decomposers break down dead organic matter. Human activities, for example, the burning of fossil fuels, also release carbon into the atmosphere. The fixed carbon enters the geosphere when dead organic matter gets buried and transformed into fossil fuels. This process may take millions of years.
3.Nitrogen Cycle
Nitrogen exists in the atmosphere in the gaseous form. Although it comprises approximately 78 percent of the atmosphere, getting nitrogen into a living organism is complex. Free-living and symbiotic bacteria convert atmospheric nitrogen into ammonia (nitrogen fixation) and then into nitrites and nitrates, a form usable by plants. When heterotrophs eat plants, they acquire nitrogen in their bodies.
Nitrogen is released back into the atmosphere when certain bacteria obtain energy by converting nitrates into nitrogen gas (denitrification).
4. Phosphorus Cycle
Phosphorus exists in nature as a phosphate ion (PO43-). It is introduced into the water bodies as agricultural runoff and naturally from the weathering of phosphate-containing rocks. Volcanic ash, aerosols, and mineral dust also act as significant phosphate sources forming sediments in the ocean. These sediments then move to land over geologic time by upliftment of the earth’s surface.
Plants take up organic phosphates from soil and underground water and convert them to inorganic forms. This inorganic matter is consumed by animals and incorporates phosphates into their bodies. Phosphorous is released back into the soil and water bodies when microorganisms decompose the dead organic matter of plants and animals.
Learn more about the phosphorous cycle in our phosphorous cycle article.
5. Sulfur Cycle
Sulfur exists in the atmosphere as sulfur dioxide (SO2), which enters the atmosphere by decomposing organic molecules, volcanic activity, and burning of fossil fuels.
Atmospheric sulfur returns into the soil through precipitation as sulfuric acid (H2SO4), direct fallout from the atmosphere, weathering of sulfur-containing rocks, and geothermal vents. Plants then take up these soil sulfates (SO42-) through their roots to use them during their growth. This way, sulfur makes its way into the food web. Sulfur is released into the atmosphere as H2S once those plants die and are decomposed in the soil by microorganisms. Soil sulfur then enters the water bodies through surface runoff and from underwater geothermal vents.
Why are Biogeochemical Cycles Important
Biogeochemical cycles help explain how our planet earth conserves matter and uses energy. They are crucial to ecosystem functioning because they move elements through it so that transformation can happen and elements can be stored and recycled. Furthermore, the biogeochemical cycles establish a connection between the living and nonliving things on earth.
Which Biogeochemical Cycles are Essential for Life
Living organisms use all the six most common elements as part of their lifecycle. Hydrogen and oxygen combine to form water, which is essential for all living organisms. The human body is made of almost 60 percent water, and human cells are more than 70 percent water. The water cycle is thus high on the importance list.
However, humans cannot live by water alone. Some of the other key elements are also essential to keep our bodies working and are part of the biogeochemical cycles. They are given below.
- Carbon Cycle: Along with hydrogen and oxygen, carbon forms the building block of atoms in all living organisms. It is found in fossil fuels, which human beings are economically dependent on.
- Nitrogen Cycle: It forms a component of nucleic acids and proteins
- Phosphorous Cycle: It is used to make nucleic acids and phospholipids that make up biological membranes. As calcium phosphate, it forms the supportive components of our bones.
- Sulfur Cycle: Sulfur is crucial in determining the shape of proteins.
These cycles do not happen in isolation but are interconnected with one another. The water cycle, among all others, is an essential driver of all other biogeochemical cycles. For example, the movement of water is crucial for nitrogen and phosphorous leaching into the water bodies.
Finally, the biogeochemical cycles keep matter moving and make it helpful for living organisms, thus keeping the biosphere balanced and contribute to the earth’s sustainability.
FAQs
Ans. The phosphorus cycle does not have an atmospheric component and differs from other biogeochemical cycles like water, nitrogen, and carbon.
Ans. The oxygen cycle and the carbon cycle depend directly on photosynthesis.
Ans. The phosphorous cycle is least dependent on the biotic process.
Ans. Deforestation adversely affects the water and the carbon cycle. It decreases the amount of transpiration and increases the amount of carbon dioxide in the atmosphere due to a decrease in photosynthesis.
Ans. Iron is abundantly available in the earth’s crust.The iron biogeochemical cycle is the cycle of iron through the atmosphere, hydrosphere, biosphere, and lithosphere.
-
References
Article was last reviewed on Thursday, February 2, 2023