Cellulose
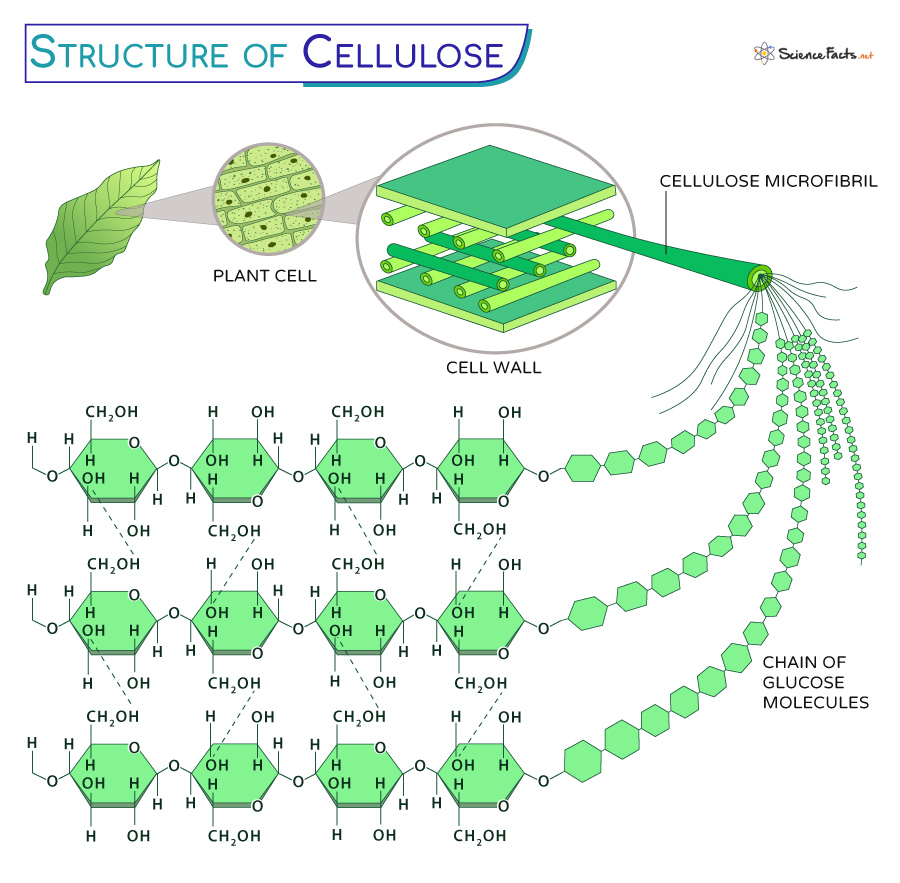
Cellulose (C6H10O5)n is an organic compound, the most abundant biopolymer on Earth. It is a complex carbohydrate with a linear chain of tens to hundreds to several thousand D-glucose units.
It is the principal structural component of plant and algal cell walls. While animals do not produce them, many microorganisms, like bacteria, also make biofilms. Humans cannot digest cellulose as they lack the enzymes that can break down the β-acetal linkages of cellulose.
Anselme Payen discovered cellulose from plant matter in 1838 and determined its chemical formula. It was first used to produce the first successful thermoplastic polymer, celluloid. Cotton is the purest natural form of cellulose, consisting of over 90% cellulose. In 1920, Hermann Staudinger discovered the chemical structure of this macromolecule.
Cellulose plays a vital role in providing structure and strength to plants. It also finds great importance in the industry.
Structure and Properties of Cellulose
- Cellulose is an example of a linear polysaccharide composed of repeating glucose monomers linked by β-1,4-glycosidic bonds, unlike starch, which is made of α-1,4-glycosidic bonds. The polymerization of glucose units forms long chains, which combine to create the cellulose microfibrils, a critical structural component in plant cell walls. The hydroxyl groups (-OH) on the glucose molecules form hydrogen bonds with oxygen atoms.
- The linear structure allows cellulose molecules to form strong hydrogen bonds with neighboring molecules, creating a highly stable network.
- Each glucose molecule is flipped 180 degrees during joining, compared to its adjacent glucose molecule in the chain. This alternating orientation of glucose units results in a straight, linear chain.
- Pure cellulose is odorless and flavorless. Due to numerous hydroxyl groups, cellulose is hydrophilic but insoluble in water.
- Enzymatic hydrolysis of cellulose forms glucose.
- Cellulose has both crystalline and amorphous regions in its microfibrils. The highly ordered crystalline parts provide strength and resistance to deformation, while the amorphous regions offer flexibility and allow for enzymatic degradation.
Types of Cellulose
Four different polymorphs of cellulose exist. They are cellulose I, II, III, and IV. Cellulose I is naturally occurring which exists as Iα and Iβ. Cellulose found in bacteria is enriched in Iα, while in higher plants, it exists as Iβ.
In contrast, cellulose II exists in regenerated cellulose fibers. The conversion of cellulose I to cellulose II is irreversible. Cellulose III is obtained by treatment of cellulose I or II with amines. Similarly, cellulose IV forms after treatment of cellulose III with glycerol at very high temperatures.
Functions of Cellulose
Cellulose serves many functions in living organisms.
- Provides Rigidity to Plants: Cellulose primarily offers structural support to plant cells. It forms a robust and rigid cell wall, giving plant cells their characteristic shape and preventing them from collapsing under pressure.
- Regulates Water Uptake and Transportation: It also helps regulate water uptake in plants and transportation. It helps maintain the osmotic pressure in plant cells, ensuring proper water balance and turgidity.
- Provides Growth and Development: Cellulose is involved in cell growth and expansion during plant development. As the plant grows, new cellulose microfibrils develop, increasing the size of the cell wall and enabling cell elongation.
- Protects from Pathogens: The cellulose cell wall is a physical barrier, protecting plant cells from external threats, such as pathogens and herbivores.
- Produces Biofilms: Some bacteria secrete cellulose to form biofilms that help microorganisms to attach and organize into colonies.
Commercial Uses of Cellulose
One of Earth’s most abundant and renewable biomaterials, cellulose, finds various commercial applications. Its unique properties, such as biodegradability, strength, and chemical reactivity, make it an attractive choice for sustainable and eco-friendly products.
- The most significant commercial application of cellulose is paper and paperboard production. Wood pulp, which contains a high percentage of cellulose, is processed to extract the cellulose fibers. These fibers are then converted into pulp to manufacture various paper products, including newspapers, books, packaging materials, and cardboard.
- Cellulose-based fibers are used in the textile industry to produce fabrics like rayon, modal, and lyocell. These fibers are derived from cellulose obtained from plant sources such as wood, bamboo, or cotton.
- Derivatives, such as cellulose acetate and methylcellulose, are used to produce biodegradable films and coatings, which are used to pack food items.
- Derivatives, like microcrystalline cellulose, are widely used as excipients in the pharmaceutical industry, serving as bulking agents in tablets and capsules.
- Cellulosic biomass, including agricultural residues, wood chips, and energy crops, is a potential feedstock for biofuel production.
- Many cellulose-derived polymers are biodegradable and serve as renewable resources. They tend to be non-toxic. Some common cellulose derivatives are celluloid, cellulose acetate, cellulose triacetate, nitrocellulose, methylcellulose, cellulose sulfate, and rayon.
- It is used in the construction industry to produce cellulose insulation.
Starch vs. Cellulose
Starch and cellulose are structurally similar yet functionally distinct polysaccharides found abundantly in the plant kingdom. The critical differences between starch and cellulose are listed in the table below.
| Basis | Starch | Cellulose |
|---|---|---|
| 1. Structure | It is a polysaccharide composed of two types of glucose molecules: amylose and amylopectin.Amylose is a linear chain of glucose units connected by α-1,4-glycosidic bonds, while amylopectin is a branched chain with both α-1,4-glycosidic and α-1,6-glycosidic bonds. | It is a linear polysaccharide composed of glucose units linked together by β-1,4-glycosidic bonds. The alternating orientation of glucose units results in straight, unbranched chains. |
| 2. Main Function | It is the plant’s primary energy storage form. It is broken down into glucose in times of need. | It is a critical component of plant cell walls, providing structural support and rigidity to plant cells. |
| 3. Digestibility | It is easily digestible by humans and many animals due to the presence of α-amylase enzymes in their digestive systems. | It is indigestible by humans and most animals due to the absence of enzymes that break the β-1,4-glycosidic bonds. |
| 4. Solubility | Partially soluble in water. | Insoluble in water and other common solvents. |
Amylose vs. Cellulose
The critical differences between amylose and cellulose are listed in the table below.
| Basis | Amylose | Cellulose |
|---|---|---|
| 1. Structure | It is a linear polysaccharide consisting of glucose units connected by α-1,4-glycosidic bonds. | It comprises glucose units arranged in a linear chain linked by β-1,4-glycosidic bonds. |
| 2. Main Function | It acts as a storage polysaccharide in plants. | It is a structural component in plant cell walls, providing strength, rigidity, and protection to plant cells. |
| 3. Digestibility | It is digestible by both humans and animals. | It is indigestible by most animals, including humans. |
| 4. Solubility | It is partially soluble in water. | It is insoluble in water. |
Glycogen vs. Cellulose
| Basis | Glycogen | Cellulose |
|---|---|---|
| 1. Structure | It comprises glucose units linked by both α-1,4-glycosidic bonds in the linear chains and α-1,6-glycosidic bonds at branching points. | It consists of linear chains of glucose units linked together by β-1,4-glycosidic bonds. |
| 2. Main Function | It acts as a short-term energy storage polysaccharide in animals. | Provide structural support to plant cells. |
| 3. Digestibility | It is highly digestible by animals, including humans. | It is indigestible by most animals, including humans. |
| 4. Occurrence | It is mainly found in animals and certain fungi and bacteria. | It is predominantly found in the cell walls of plants. |
-
References
Article was last reviewed on Wednesday, September 13, 2023