Contact Angle
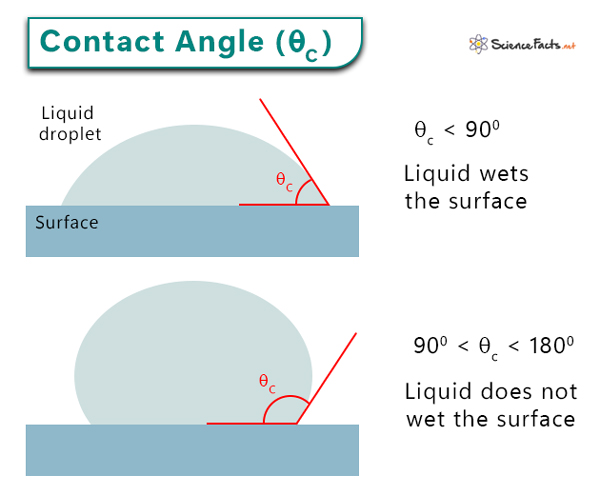
Contact angle is a fundamental concept in surface science and engineering. It measures how a liquid spreads on a solid surface, providing insights into wettability. Contact angle is the angle between a liquid droplet and a solid surface formed at the interface between solid, liquid, and gas. More importantly, contact angle allows researchers to study the interactions between these three phases. The cohesive and adhesive forces describe these interactions.
Principle
Cohesive forces occur between molecules of the same kind, typically that of the liquid. These forces are like hydrogen bonds and Van der Waals forces. Adhesive forces occur between molecules of different kinds, such as between liquid and solid molecules. These are mechanical and electrostatic forces. The balance between the two forces determines the contact angle.
- When the contact angle is small, cohesive forces are weaker than adhesive forces. The liquid molecules interact more with the solid molecules than with themselves. The droplet spreads across the surface. For example, water spreads over a glass.
- When the contact angle is large, cohesive forces are stronger than adhesive forces. The liquid molecules interact more with each other than with the solid molecules. The droplet beads up and does not wet the surface. For example, water forms round droplets on a lotus leaf. Mercury forms droplets when deposited over glass or metal.
Surfaces that attract water are hydrophobic surfaces (for example, glass). Surfaces not attracting water are known as hydrophilic surfaces (for example, lotus leaves).
Balanced Force Equation
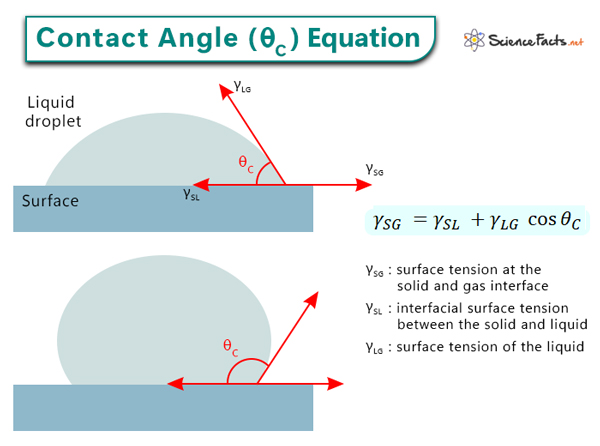
Young’s equation is a fundamental equation in surface science that establishes a relationship between the contact angle of a liquid droplet on a solid surface and the interfacial surface tensions. This equation represents the equilibrium between the liquid, solid, and vapor phases at the three-phase contact line. Since the three-phase boundary is not moving, Young’s equation can only measure the static contact angle.
Mathematically, Young’s equation is expressed as follows:
γSG = γSL + γLG ˑ cos θC
Where
θC is the contact angle
γSG is the surface tension at the solid and the gas interface. It is also the free surface energy of the solid and measures how strongly the solid molecules attract each other.
γSL is the interfacial surface tension between the solid and the liquid.
γLG is the liquid’s surface tension. It is also the free surface energy of the liquid and expresses how strongly the liquid molecules attract each other.
Rearranging the equation, we get
cos θC = (γSG – γSL)/γLG
This equation provides some helpful information:
- If γSG > γSL, cos θC is positive and θC < 90°. The droplet wets the surface. It occurs with a high surface energy solid such as a metal or a low surface tension liquid such as acetone.
- If γSG < γSL, cos θC is negative and 90° < θC < 180°. The droplet does not wet the surface and beads up. It occurs with a low surface energy solid such as polystyrene or a high surface tension liquid such as mercury.
Young’s equation establishes that contact angles and surface energies or surface tensions strongly connect to adhesion. By examining the behavior of a liquid droplet on a solid surface, researchers can leverage the relationship between this observation and the surface energy of the solid.
Factors Affecting Contact Angle
Several factors influence the contact angle between a liquid droplet and a solid surface. Understanding these factors is crucial for accurately measuring and interpreting contact angle data. The following vital factors significantly impact contact angle measurements:
- Surface Tension: Higher surface tension tends to result in a larger contact angle, leading to droplets that bead up on the surface. On the contrary, lower surface tension promotes wetting, resulting in droplets that spread out.
- Surface Roughness: The roughness of a solid surface affects the contact angle by altering the apparent contact area. A rough surface provides more contact points for the liquid droplet, leading to increased wetting and a smaller contact angle. In contrast, a smooth surface reduces the contact area, resulting in less wetting and a larger contact angle.
- Chemical Composition: The chemical composition of the solid surface and the liquid can significantly influence the contact angle. The interactions between the molecules at the interface determine whether the liquid spreads or beads on the surface. If the liquid and surface have similar chemical properties, the contact angle tends to be smaller, indicating better wetting. On the contrary, unlike chemical properties result in a larger contact angle and limited wetting.
- Temperature and Pressure: Temperature and pressure can influence the contact angle by affecting the intermolecular forces within the system. Temperature changes can alter the surface tension and the contact angle. Similarly, variations in pressure can impact wetting behavior by affecting the equilibrium between the liquid, solid, and vapor phases.
How to Measure Contact Angle
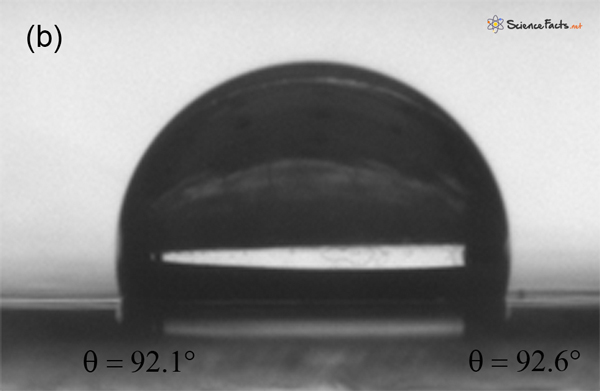
The principal technique used to measure contact angles is the sessile drop method, which involves estimating the contact angle by averaging the angles at two points on opposite sides of a stationary drop. Typically, these angles are determined either directly by drawing a tangent line at the point where the three phases (liquid, solid, and vapor) meet or indirectly through drop shape analysis. This method requires a flat, smooth surface and a consistent spherical drop shape to obtain precise and accurate results. A benchtop contact angle goniometer or an optical tensiometer is a commonly used laboratory instrument for measuring contact angles.
Applications
Contact angle measurements are widely used in materials science, chemistry, biology, and medicine. By measuring the contact angle, researchers can determine the wettability of different materials, which is essential for understanding their performance in various applications. For example, in the development of coatings or paints, knowing the contact angle helps to improve their adhesion to surfaces. Industries such as cosmetics and textiles rely on accurate contact angle data to assess product performance related to wetting ability, adhesion strength, durability, and surface cleanliness.
-
References
Article was last reviewed on Thursday, July 13, 2023