Glucose-Alanine Cycle
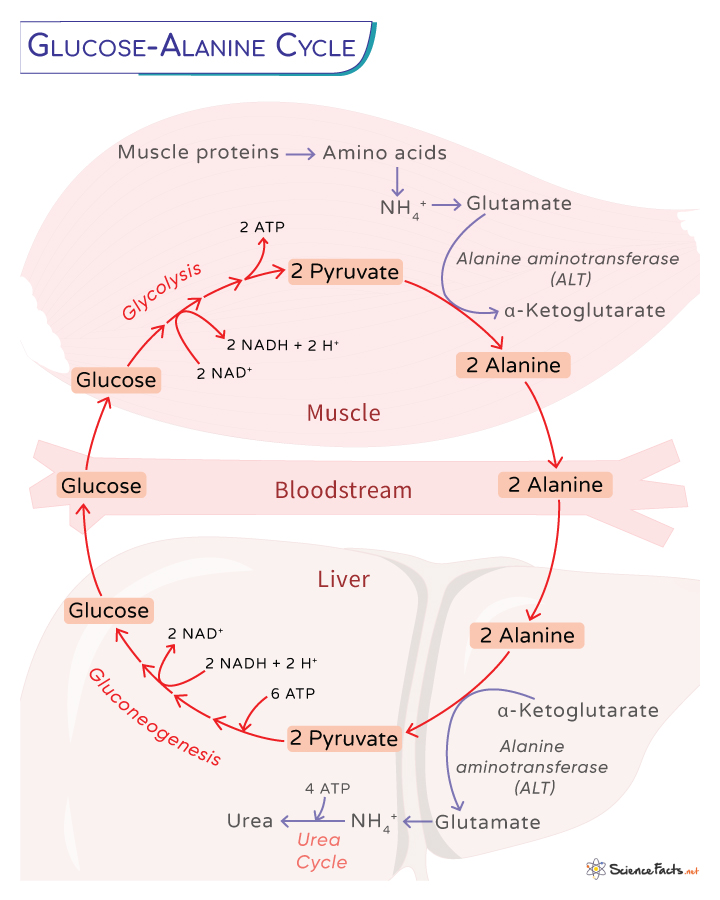
The glucose-alanine cycle, also known as the Cahill cycle or alanine cycle, is a biochemical pathway in which muscle proteins are degraded to produce more glucose for energy during conditions of intense muscular stress. Like the Cori cycle, it involves multiple organs and tissues, including muscle cells, the bloodstream, the liver, and even immune system cells, such as lymphoid organs. This cycle requires the presence of the enzyme alanine transaminase (ALT), which is exclusively found in these tissues.
Mallette, Exton, Park, and Felig et al. proposed it for the first time between 1969 and 1970 in the skeletal muscle.
The Steps of the Glucose-Alanine Cycle
The cycle occurs when our muscles are under intense stress, such as fasting or due to prolonged exercise. During those periods, the muscle proteins are catabolized to amino acids much faster than their synthesis rate, forming glutamate through transamination. Such reactions are catalyzed by enzymes called aminotransferases or transaminases.
For example, aspartate transfers its amino group to glutamate when it reacts with oxaloacetate, forming α-ketoglutarate.
Aspartate + Oxaloacetate ⇄ α-Ketoglutarate + Glutamate
Some synthesized amino acids are used during metabolism, while others are used during glucose synthesis through gluconeogenesis.
In the Skeletal Muscles
The newly formed glutamate can be converted to glutamine by the cytosolic enzyme glutamine synthetase for transport to the liver.
Glutamate + NH4+ + ATP → Glutamine + ADP + Pi
Alternatively, the glutamate transfers its amino group to pyruvate through the enzyme alanine aminotransferase (ALT), forming alanine and α-ketoglutarate.
Glutamate + Pyruvate ⇄ Alanine + α-Ketoglutarate
In the Bloodstream
Alanine leaves the muscle cells and moves through the circulatory system to the liver. The rate at which alanine is formed through transamination of pyruvate and sent to circulation is proportional to the synthesis of pyruvate inside the cell.
In the Liver
Once inside the liver, a hepatic ALT catalyzes a transamination reaction, converting alanine to pyruvate. Alanine acts as an amino group donor and α -ketoglutarate as an alpha-keto acid acceptor.
Alanine + α-Ketoglutarate ⇄ Glutamate + Pyruvate
Meanwhile, glutamate dehydrogenase in the mitochondrial matrix catabolizes glutamate into ammonium, which is then used in the urea cycle, and the α-ketoglutarate enters the Krebs cycle. It is an anaplerotic reaction linking amino acid metabolism with the Krebs cycle.
Glutamate + H2O + NAD+ ⇄ α-Ketoglutarate + NH4+ + NADH + H+
The pyruvate can have different metabolic fates. It can be oxidized for ATP synthesis, leaving the glucose-alanine cycle, or can enter the gluconeogenesis pathway to produce glucose, which helps to continue the cycle.
In an alternate reaction, glutamate can also react with oxaloacetate to form aspartate and alpha-ketoglutarate, catalyzed by aspartate aminotransferase.
Glutamate + Oxaloacetate ⇄ Aspartate + α-Ketoglutarate
The glucose-alanine cycle is less productive than the Cori cycle in energy production as the byproduct urea formed during the energy production from alanine requires removal from the system. This urea removal is an energy-dependent process requiring 3ATP to be hydrolyzed to 2ADP and 1AMP. However, unlike the Cori cycle, the NADH is stored for use in the electron transport chain.
Purpose of the Glucose-Alanine Cycle
The glucose-alanine cycle is a crucial adaptation that allows your body to maintain adequate energy levels during strenuous activities. In such situations, this cycle enables glucose to be rapidly transported from the liver to the muscles for a continuous energy supply.
During intense exercise, our muscles produce lactate as a byproduct of anaerobic metabolism. Accumulation of lactate leads to muscle fatigue. The glucose-alanine cycle aids in removing lactate from the muscles by transporting it to the liver, where it is converted back into glucose.
-
References
Article was last reviewed on Thursday, November 30, 2023