Hypotonic Solution
What is a Hypotonic Solution
A solution is considered hypotonic if it contains a lower solute concentration or higher water content than another solution. The Greek word ‘hypo’ stands for ‘under’ or ‘low’, whereas ‘tonic’ is derived from ‘tonicity’, which means ‘relative concentration of a solution’.
It is a relative term and can only be measured by comparing two solutions. Generally, it is compared to the cytosolic fluid (the fluid present inside a cell).
Some widely used hypotonic solutions are freshwater, tap water, and normal saline (0.45% NaCl).
Why Is It Important In Cells
Cells always strive to maintain the equilibrium between their internal and external environments.
If the cell interior has a lower solute concentration (hypotonic), it confers that its extracellular medium has a higher concentration of solutes (hypertonic). Several essential cellular constituents, such as ions, electrolytes, and water, generally diffuse along their concentration gradients, i.e., from higher to lower concentration.
When the cell interior is hypotonic, water moves out of the cell, equalizing the solute concentration inside the cell and its surroundings.
Living cells can retain the rigid structure due to this regulation.
What Happens to a Cell When Placed in a Hypotonic Solution
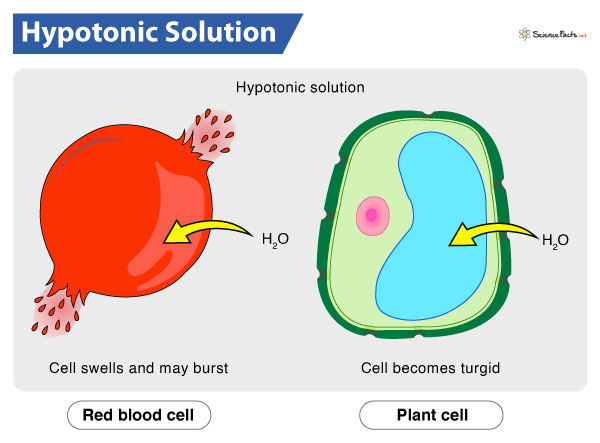
When we place a cell in a hypotonic solution, water gushes into the cell.
As already stated, the hypotonic solution contains less solute and more water than the cell interior. So, following the law of osmosis, water moves from its higher concentration to its lower concentration across the semi-permeable membrane. Consequently, the cytoplasm gains water, and the cell expands. As the cell membrane is freely permeable to water molecules, it allows the entry of water by endosmosis. That is why hypotonic solutions are crucial to the cells in case of cell dehydration, as it allows the entry of water into the cell.
If water influx continues past the permissible level, the osmotic balance of the cell might get disturbed. As a result, the cell will fail to hold the pressure, and its membrane will stretch to the limit that it lyses or bursts.
What Happens to RBCs in a Hypotonic Solution
RBCs, the oxygen carrier of blood, get bloated up and eventually burst when exposed to a hypotonic solution. Under any circumstances, it can hamper the oxygen transport in the body.
Examples of Hypotonic Solution
In Plants and Fungi
Plants and fungi perform better in hypotonic conditions. So, they monitor and control their cells’ surroundings to ensure that the environment is always hypotonic compared to their cells. They either constantly keep on cycling solutes, or transpire through leaves, to keep the contents of their cells filled with water.
Due to the continuous influx of water, the cells become swollen and stiff (turgid). The pressure thus generated, i.e., the turgor pressure, provides structural rigidity to plants, which helps them to stand upright. They also utilize this pressure for transporting water throughout their body, from roots to the top of the plants. The cell wall and vacuole play a significant role in this. The vacuole absorbs the excess water. On the other hand, the cell wall creates an opposite wall pressure, thus preventing the plant cell from bursting.
Similarly, in fungi, their rigid chitinous cell wall exerts wall pressure upon entry of water, which helps them to maintain their shape and structure.
In Animals
As animal cells lack a cell wall, they may burst due to an excessive inflow of water when kept in a hypotonic medium. So, they undergo osmoregulation, a process to maintain their internal solute concentration. Thus the cell remains plump and healthy. For this reason, animal cells perform better in isotonic solution rather than hypotonic.
Let us discuss some examples:
- As protists, such as amoeba, and paramecia do not possess any cytoskeleton or cell wall, they retain their rigid structure by regulating their tonicity. They generally dwell in a hypotonic environment, which causes a continuous influx of water. To maintain the cell structure and prevent themselves from bursting, they own a specialized vacuole, called a contractile vacuole, which helps them expel the excess water.
- In marine animals, such as sharks, and sea turtles, the seawater is already hypertonic to their cell sap. So, they maintain an even more hypertonic concentration inside their cells by removing the excess salt using their salt glands. As a result, the external environment becomes relatively hypotonic, enabling them to survive in the seawater.
- Freshwater fishes like catfish and trout are adapted to live in a hypotonic environment by continuously flushing out excess water from their body.
- In the human body, kidneys maintain the osmotic pressure of blood through extensive filtration and purification. It helps to regulate the water levels and mineral ions (salt) in the blood.
FAQs
Ans. Yes, water is a typical example of a hypotonic solution, although it is based on the solution to which it is compared. Distilled water being a pure solvent, is always hypotonic compared to an aqueous solution containing any amount of solute.
-
References
Article was last reviewed on Friday, December 17, 2021