Lac Operon
The lactose (lac) operon is a group of genes involved in lactose metabolism. They are found in bacteria, most notably Escherichia coli (E. coli), that use lactose as the alternate energy source when glucose is absent.
The bacteria express the lac operon genes to use lactose, which encodes key enzymes for lactose uptake and metabolism. For performing these functions and activating the lac operon, the following two conditions are necessary:
- Presence of lactose
- Absence of glucose
However, measuring the concentrations of lactose and glucose and activation of lac operon transcription are regulated by two proteins: lac repressor and the catabolite activator protein (CAP).
Structure of lac Operon
The lac operon consists of the following components:
1. Structural Genes: The three structural genes in a lac operon are:
- lacZ: It encodes β-galactosidase, an enzyme that breaks down lactose into glucose and galactose.
- lacY: It encodes lactose permease, a protein that transports lactose into the cell.
- lacA: It encodes transacetylase, an enzyme involved in lactose metabolism.
2. Promoter (lacP): It is the region where RNA polymerase, the enzyme responsible for transcribing DNA into RNA, binds to initiate the transcription of the structural genes.
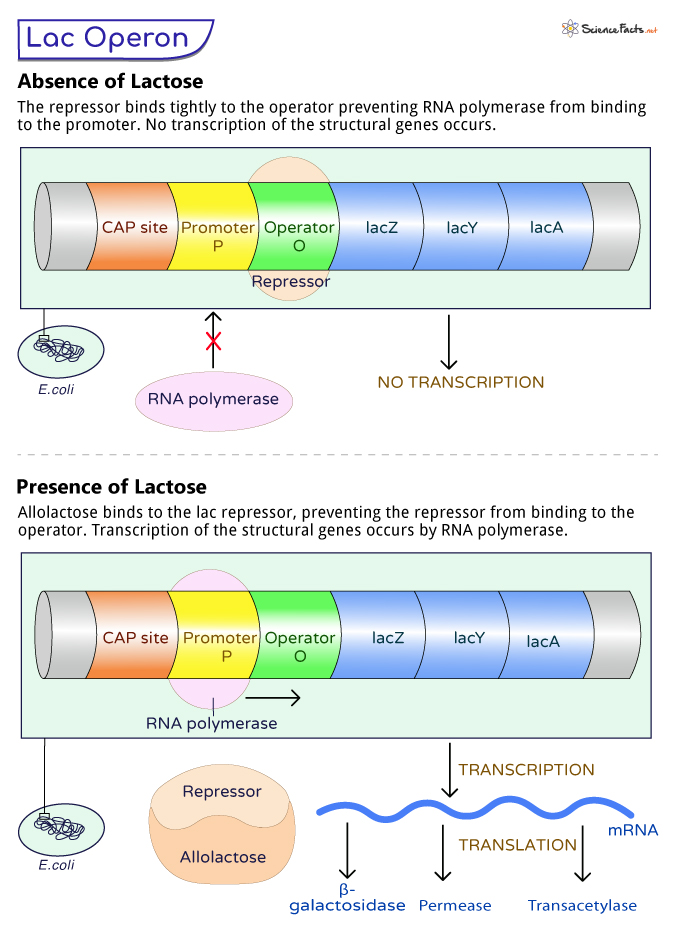
3. Operator (lacO): It is the on/off switch of the lac operon. When the lac repressor protein binds to this region, it prevents RNA polymerase from transcribing the structural genes.
4. Regulator Gene (lacI): It encodes the lac repressor protein, a key player in controlling the operon. The lac repressor binds to the operator, blocking the expression of the structural genes in the absence of lactose.
How is the Lac Operon Regulated
The working of the lac operon depends on the presence or absence of lactose in the cell.
When Lactose is Absent
During the absence of lactose, the lac operon remains inactive. Encoded by the regulator gene (lacI), the lac repressor protein is synthesized and binds tightly to the operator region (lacO). This region is between the promoter (lacP) and the structural genes (lacZ, lacY, and lacA).
The lac repressor acts as a roadblock, preventing RNA polymerase, the enzyme responsible for transcribing DNA into RNA, from accessing the structural genes. Without RNA polymerase access, the genes cannot be transcribed into messenger RNA (mRNA), and the production of enzymes involved in lactose metabolism does not occur.
When Lactose is Present
As we know, the lac operon operates only when lactose is present. As lactose enters the cell, it is converted to allolactose, an isomer of lactose by the enzyme β-galactosidase, encoded by the lacZ gene.
Allolactose acts as an inducer molecule in the lac operon system. It binds to the lac repressor protein, causing a change in the protein’s shape and making it unable to attach to the operator region. This release of the lac repressor from the operator region removes the physical blockage, allowing RNA polymerase to access the promoter and initiate transcription.
RNA polymerase binds to the promoter, as the lac repressor is no longer bound to the operator region. It transcribes the structural genes (lacZ, lacY, and lacA) into mRNA that produces enzymes β-galactosidase, lactose permease, and transacetylase.
The newly synthesized enzymes actively participate in lactose metabolism. β-galactosidase converts lactose into glucose and galactose, which the bacterium can use as a source of energy. Lactose permease facilitates the entry of lactose into the cell, maintaining a continuous supply for metabolism. Transacetylase’s role is not entirely known, but it may be involved in detoxifying some lactose-related byproducts.
As lactose levels decrease in the environment and are used up by the bacteria, the concentration of allolactose also decreases. The lac repressor, no longer bound by allolactose, can reattach to the operator region, effectively shutting down the lac operon and preventing unnecessary synthesis of lactose-metabolizing enzymes until lactose becomes available. IPTG or Isopropyl β-D-1-thiogalactopyranoside is an artificial analog of allolactose that is also sometimes used to control the functioning of the lac operon.
The lac operon is thus an inducible operon.
How does Glucose Affect the Lac Operon
In the presence of lactose, the repressor loses its DNA-binding ability, allowing RNA polymerase to bind to the promoter and transcribe the lac operon.
However, it is more complicated. RNA polymerase alone cannot bind well to the promoter. It might make a few transcripts but will stop unless it gets extra help from catabolite activator protein (CAP). CAP is not active on its own. Its activity is regulated by the concentration of cyclic AMP (cAMP) in the cell, which depends on the glucose concentration in the cell.
When the Glucose Concentration is Low
When glucose concentration is low, cAMP is produced, which binds to CAP, forming the CAP- cAMP complex. The cAMP-CAP complex then binds to a specific site upstream of the promoter, facilitating the recruitment of RNA polymerase to the promoter. CAMP-CAP attaching to its location near the promoter region results in a conformational change in the DNA, allowing RNA polymerase to bind more effectively to the promoter.
This interaction between cAMP-CAP and RNA polymerase increases the transcription of the three structural genes (lacZ, lacY, and lacA) into mRNA. Thus, the lac operon is positively regulated by the absence or even low concentration of glucose.
When the Glucose Concentration is High
In contrast, when glucose concentration is high, no cAMP is produced. Thus, CAP cannot bind to the CAP binding site, and the transcription continues slowly.
Here is the summary of the expression of the lac operon as required by the cell:
| Conditions | Regulation of Lac Operon |
|---|---|
| 1. Glucose present, lactose absent | No transcription |
| 2. Glucose present, lactose present | Low-level transcription |
| 3. Glucose absent, lactose absent | No transcription |
| 4. Glucose absent, lactose present | Strong transcription |
-
References
Article was last reviewed on Tuesday, August 29, 2023