Proteolysis
Proteolysis is the process of breaking proteins into peptides and amino acids in living organisms. It is a prolonged process catalyzed by specific enzymes called proteases, which are present in bacteria and plants but most abundant in animals. Without these enzymes, the cleavage of protein bonds may take hundreds of years.
It is a vital process in humans and animals that helps to maintain cellular functions such as regulation of metabolism, maintaining cell structure, and transportation. Proteolysis is also a key player in causing some diseases. In addition, it also has a few industrial applications, such as food processing and stain removal.
How Proteolysis Works with Steps
The process of enzymatic proteolysis is a series of irreversible steps causing changes in protein structure. The primary reaction involved in peptide bond-breaking is the hydrolysis of the amide bond, which is carried out in the following ways:
Step 1: Initially, globular proteins are broken down into smaller protein fragments and polypeptides. This mechanism in eukaryotic cells mainly follows a lysosomal or proteasomal pathway. Lysosomes non-selectively degrade long-lived protein chains. Proteasomes deal with short-lived or abnormal proteins in a very selective manner.
Two main lysosomal proteases control the breakdown of long-lived proteins; calpains and cathepsins. Calpains cause limited hydrolysis of myofibrils in the early stages of cell breakdown. Cathepsins act in the later stages of intercellular protein degradation to convert them into smaller proteins and polypeptides.
Step 2: A small protein called ubiquitin mediates the breakdown of short-lived proteins via proteasomes. It marks the protein chains that must be broken by covalently attaching to the cleavage site. The proteasome then frees the ubiquitin by cleaving the peptide bond. The newly-freed ubiquitin then marks another place, and the process continues.
Step 3: Di- and tri-peptidyl peptidases hydrolyze the protein fragments and polypeptides obtained from the above steps, generating smaller peptides.
Step 4: These peptides are finally broken down into free amino acids by the action of aminopeptidases, dipeptidases, and carboxypeptidases.
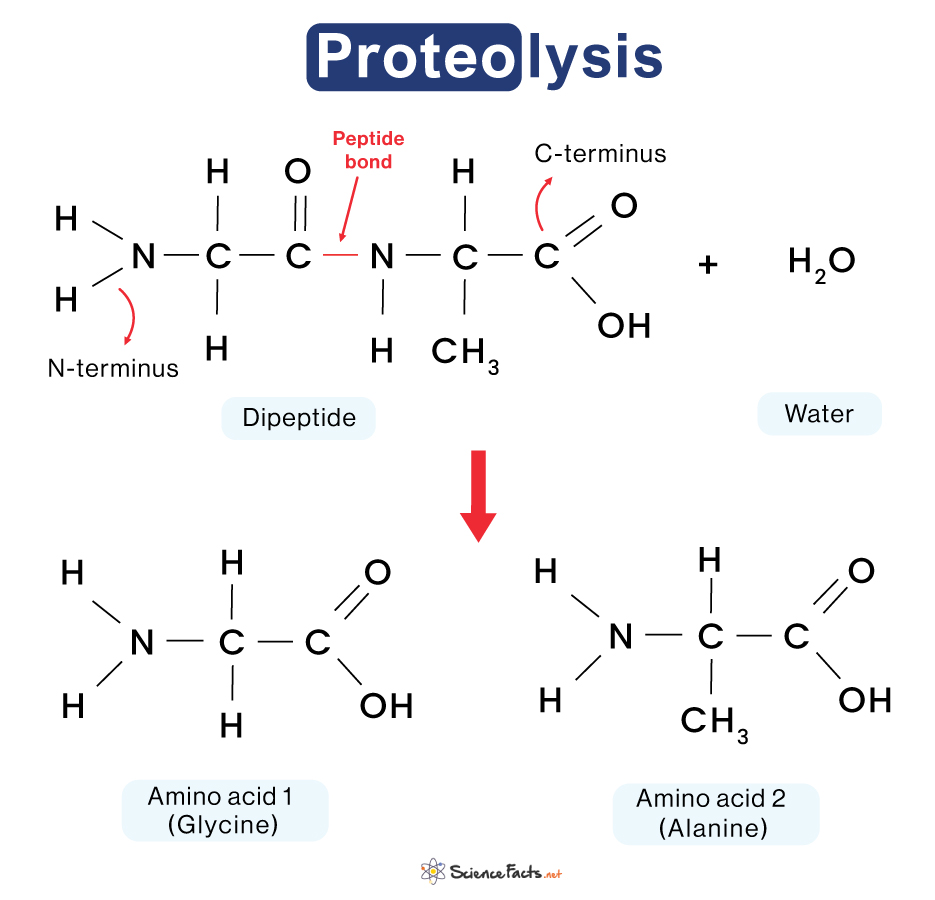
The figure below illustrates the hydrolysis of amine bonds in proteolysis.
Non-Enzymatic Processes
Apart from lysosomal or proteasomal degradation, proteins can also degrade via non-enzymatic pathways. Usually, high temperature or low pH can trigger the cleavage of peptide bonds. Acids such as trifluoroacetic acid and formic acids readily hydrolyze peptide links to give the constituent amino acids.
The energy of the peptide bond gets released by proteolysis, which forms the basis of many physiological functions.
Functions of Proteolysis
The changes that occur in proteins due to proteolysis affect both intracellular and extracellular signaling pathways. It can have a wide range of implications for living organisms.
Regulation of Cellular Functions
Proteolysis plays a significant role in the regulation of cellular functions. It helps initiate the process of cell division by activating and deactivating certain enzymes, transcription factors, and cell receptors. It can also be one of the pathways used for apoptosis or regulated cell death.
Post-Translational Modifications
Processing proteins synthesized in ribosomes is essential for their maturation and proper functioning within a cell.
The precursor to the final form of the protein is called the proprotein, which may be synthesized initially as preproproteins. They may be obtained by signaling peptides, removing the N-terminal methionine, or converting nonfunctional or inactive proteins into an active form.
Protein Degradation
Protein degradation can be either intracellular or extracellular. The amino acids obtained from proteolytic degradation inside the cells are reused to make new proteins. Removing damaged or abnormal proteins and enzymes prevents their accumulation, which helps to regulate cellular metabolism.
One of the most vital extracellular processes is gastric proteolysis. Here, digestive enzymes such as trypsin, pepsin, and chymotrypsin break down the large protein chains into smaller peptide fragments. These fragments are then further hydrolyzed by peptidases to yield absorbable amino acids. This entire cycle is carried out in the alimentary canal.
Proteolysis and Diseases
Abnormal proteolytic functioning may lead to several health complications and diseases. Alzheimer’s and Parkinson’s disease are related to the unregulated clumping of cellular proteins due to faulty proteolytic activity.
Some chronic inflammatory conditions, such as rheumatoid arthritis and diabetes mellitus, are thought to be caused by an excess of lysosomal activity. Pancreatitis is caused by high concentrations of proteases in the pancreas, leading to the degradation of the organ itself.
Laboratory Applications
Proteolytic studies give us a better understanding of the functioning of the human body. Laboratory studies involving the detection of protein degradation and purification of amino acids have led to medical advancements in treating many chronic diseases.
Enzymatic and non-enzymatic proteolysis finds use in industries such as food processing and stain removal. Both rely on the formation and breakage of peptide bonds. Understanding these processes can help create more targeted products and improve quality of life.
-
References
Article was last reviewed on Thursday, May 11, 2023