Conduction
Thermal conduction is the flow of thermal energy (heat) from higher to lower temperatures through molecular vibrations and collisions. Conduction occurs within an object or from a hot object to a cold object in contact with the former. It can occur in solids, liquids, and gases but is primarily observed in solids where molecules are closely packed. Heat will continue to flow until thermal equilibrium is reached.
Examples
- Warming hands by touching a hot body
- Heating one end of a metal rod
- Heating a frying pan on top of a stove
- Hot air immediately above the Earth’s surface
How is Heat Transferred Through Thermal Conduction
According to kinetic theory, matter is made of particles that are in constant random motion. This motion manifests as thermal energy, which depends on the temperature. The higher the temperature, the higher the thermal energy. The motion of particles, whether translatory or vibrational, leads to collisions. The particles transfer energy among themselves. Consequently, heat travels from a high-temperature region to a low-temperature region.
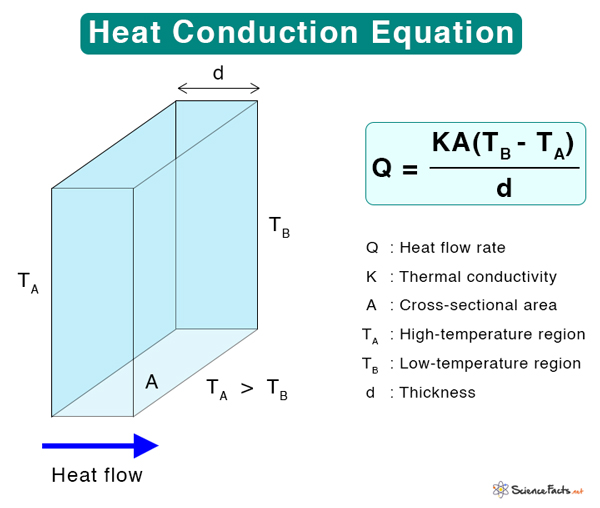
Equation
Fourier’s Law of Thermal Conduction
For thermal conduction to occur, there has to be a temperature gradient. Fourier’s law of thermal conduction states that the time rate of heat transfer through a material is proportional to the negative temperature gradient and the area through which the heat flows at right angles to that gradient. Mathematically, Fourier’s law can be written as
Where
Q: Heat transfer rate (Js-1 or W)
K: Thermal conductivity (Wm-1K-1)
A: Cross-sectional area (m2)
Suppose heat flows through a conductor of thickness d whose ends are at temperatures TA (hot) and TB (cold). The heat flowing per second through the conductor is
Thermal Conductivity
Thermal conductivity is a physical property of substances. In the above equation, suppose A = 1, d = 1, and (TA – TB) = 1. Then
K = Q
Thus, thermal conductivity is defined as the amount of heat flowing through a conductor of unit length, whose cross-section has a unit area and whose ends are at a unit temperature difference.
Metals have high thermal conductivity because their valence electrons are delocalized and can efficiently conduct heat. For example, the thermal conductivities of silver and copper are 406 Wm-1K-1 and 385 Wm-1K-1, respectively.
Insulators are poor conductors of heat. They have voids in between the atoms, which interfere with heat transfer. For example, the thermal conductivity of wood ranges from 0.04 to 0.12 Wm-1K-1. Air is a poor conductor of heat. Its thermal conductivity at 0 ˚C is 0.024 Wm-1K-1.
-
References
Article was last reviewed on Monday, January 2, 2023