Atom
What is an Atom
Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. All living organisms and nonliving objects found on Earth are made of trillions and trillions of atoms. The smaller particles that make up an atom are known as subatomic particles.
The term ‘atom’ was derived from the Greek word ‘atomos’, meaning ‘indivisible’. The ancient Greek and Indian philosophers were the first to think atom as the basic unit of all matter in the universe. In the early 19th century, scientists started understanding the atom’s structure with their inner parts in more detail. In 1926, Erwin Schrodinger proposed the current atomic model that we still use today.
There are many different atoms, each having its name, size, mass, and number of subatomic particles. They are known as elements. Thus, an atom can also be defined as the simplest structural unit of an element that retains all its properties. There are 94 natural elements and 24 artificial elements that exist today.
Structure with Parts
An atom’s size is tiny, with a diameter of 0.1 to 0.5 nanometers (1 × 10−10 to 5 × 10−10 m). Thus, they cannot be seen with our naked eye. A layer of an atom is somewhat similar to a sheet of paper.
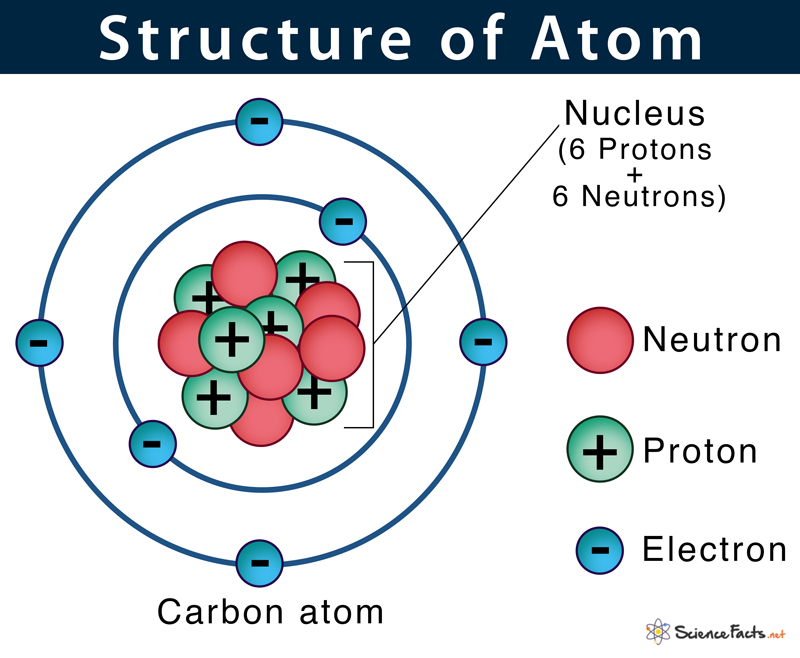
All atoms except hydrogen contain three basic subatomic particles: 1) electrons, 2) protons, and neutrons. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell.
1) Electrons
They are negatively charged particles that revolve around the nucleus in a fixed orbit. Unlike protons and neutrons, electrons are fundamental particles much smaller (almost 1800 times) in size than protons and neutrons. The standard symbol used for an electron is e or e–. British physicist J.J. Thomson discovered it in 1897.
Electrons move so fast around the nucleus that their exact location within an atom cannot be accurately determined. When the number of negatively charged electrons equals the number of positively charged protons, the atom is neutral in charge.
2) Protons
Protons are positively charged particles found within a dense region at the center of the atom called the nucleus. They were discovered by Ernest Rutherford in the year 1917 and are denoted by the symbol p or p+. Protons consist of even smaller particles called quarks and gluons.
Found tightly packed with the nucleus, they make up virtually all of the mass of an atom, along with the neutrons.
3) Neutrons
They are also found within the nucleus along with the protons in a tightly packed manner. Neutrons, similar to protons, are made of quarks and gluons. They were discovered by James Chadwick in the year 1932 and are denoted by the symbol n or n0.
Neutrons are neutral particles with no charge but have a substantial size and mass similar to a proton.
Below is a table showing the charge, mass, and location of the three sub-atomic particles:
| Name of the Particle | Symbol | Relative Charge | Location in the Atom |
|---|---|---|---|
| 1. Proton | p/p+ | +1 | Nucleus |
| 2. Neutron | n/n0 | 0 | Nucleus |
| 3. Electron | e/e– | -1 | Shell or Orbit |
Other Fundamental Particles
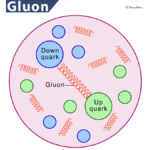
- Quarks: They are fundamental subatomic particles that makeup protons and neutrons. They were independently discovered by Murray Gell-Mann and George Zweig in 1964. However, their exact position in an atom cannot be accurately measured. Quarks are of six different types: up, down, top, bottom, charm, and strange. A proton contains three quarks (two up quarks and one down quark), whereas a neutron contains (two down quarks and one up quark).
- Gluons: Also found within protons and electrons, they act as exchange particles that help transfer strong force between quarks. John Ellis and his fellow workers discovered them in 1979.
- Neutrino: They are similar to electrons formed from nuclear reactions. Neutrino has no charge but travels at a very high speed, similar to the speed of light, and can pass through any solid object. The tremendous energy of the sun, which is obtained by nuclear fusion, releases trillions of neutrinos every second.
FAQs
Ans. There are roughly between 1078 and 1082 atoms present in the universe.
Ans. An element is made entirely of only one type of atom. In other words, an element is made of multiple atoms that are of the same type.
Ans. An atom is the smallest unit of an element, whereas a molecule consists of two or more atoms.
Ans. When an atom loses an electron, it becomes a positive ion.
-
References
Article was last reviewed on Thursday, February 29, 2024


Good
I Like the structure of atom’s and everything else.
this is a very nice information and its always useful to me here at our university mountains of the moon fort portal Uganda
Below statement is incorrect in the article:
Electrons and protons are found at the center of the atom within a dense region called the nucleus.
Instead it should be neutrons and protons are found at the center of the atom within a dense region called the nucleus
Thank you for your comment. We have edited that section.
The “Other Fundamental Particles” section states that quarks make up protons as well as electrons; it should be protons and neutrons
Thank you for your comment. We have edited that section.
Is air made of atoms
Yes. Air consist mostly of nitrogen and oxygen atoms.
There is an error in the following article excerpt:
‘There are 92 natural elements and 118 human-made elements that exist today.’
________________________
The excerpt is incorrect; there are not 92 natural elements AND 118 human made elements.
There is a total of 118 elements, of which 98 are naturally-occurring. (some people would argue there are 94 naturally-occurring, not 98, but my point is that there are 118 known elements in total).
Thank you for this article. Its simplicity is useful for new learners. 🙂
Thank you. We made the correction.