Diffusion
What is Diffusion
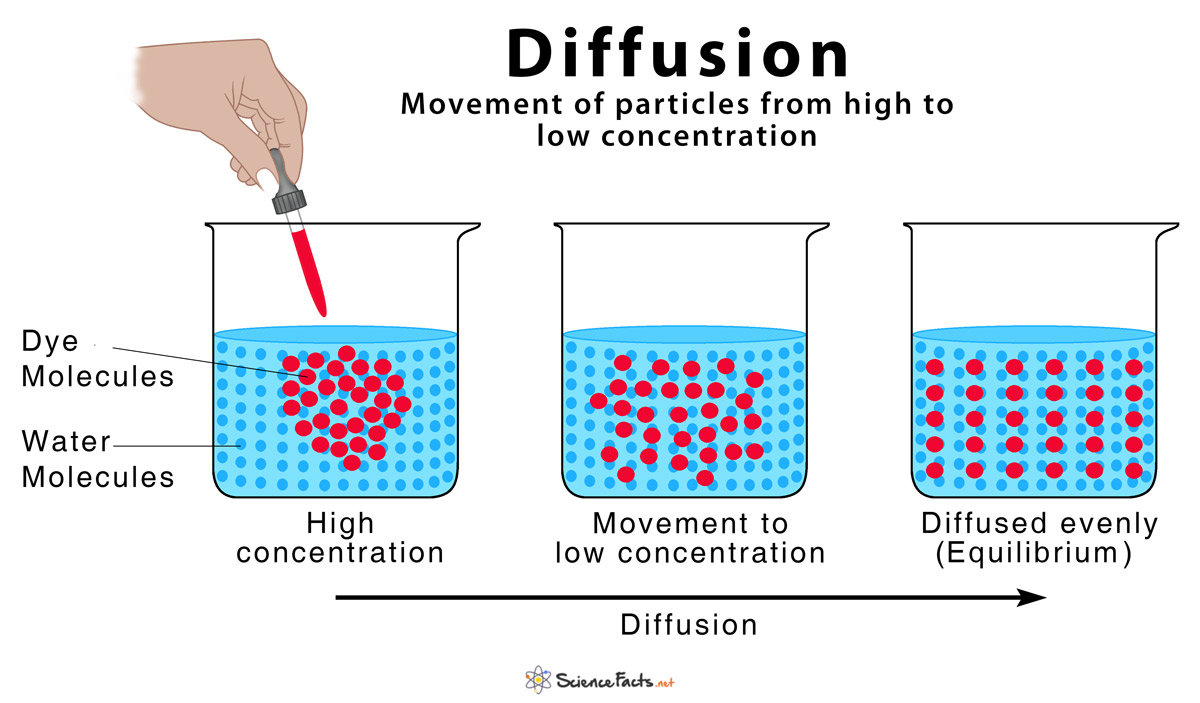
Diffusion is defined as the movement of atoms, ions, and molecules from a region of high concentration to a region of low concentration, or ‘down their concentration gradient’. The word ‘diffusion’ is derived from the Latin word, ‘diffundere’, meaning ‘to spread out’.
What Causes Diffusion and What Happens During the Process
The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Since diffusion equalizes the concentration of the substance on both sides of the region, it helps the solution to attain the state of equilibrium or minimum randomness through this process.
If Fick’s laws can describe a diffusion process, it is called a normal or Fickian diffusion, otherwise, it is named as anomalous or non-Fickian diffusion.
Examples of Diffusion
- The spreading of the odor of a scent or a perfume from the region applied to a nearby region
- Dissolving ice, sugar, salt crystals in water to form a uniform solution
Basic Characteristics of Diffusion
- It is a fast and spontaneous process
- When occurring across a biological membrane, it is a type of passive transport
- Requires no energy expenditure
- It depends upon the interaction between the diffusing material and the medium in which the diffusion occurs
- It continues until the concentration of the molecules becomes even throughout the region, or equilibrium is reached
- Takes place only when the diffusing material on both sides of the concentration gradient is fully or partially miscible
- Diffusion of any one material is independent of the diffusion of any other substance
What is the Importance of Diffusion in Cellular Processes
a) Gas exchange – Oxygen passes through the capillary membrane and enters cells to make the concentration even on both the regions
b) Respiration – The balance between oxygen and carbon dioxide within the cell is maintained by removing the excess carbon dioxide from the blood
c) Excretion – Waste products are eliminated from the body
d) Cellular Transport – Essential ions, small molecules, food, water, and minerals are taken up inside the cell
What Factors Affect Diffusion
The different factors that affect diffusion either individually or collectively are:
1) Temperature: Warmer the temperature, higher is the rate of diffusion.
2) Area of Interaction: More the surface area of interacting molecules, higher is the rate of diffusion.
3) The Extent of the Concentration Gradient: Greater the difference in concentration between the regions, higher is the rate of diffusion.
4) Diffusion Distance: Smaller the distance covered by the diffusing molecules, faster is the rate of diffusion.
5) Types of Diffusing Materials: At a particular temperature, materials with lighter atoms diffuse faster than heavier ones.
6) Particle Size: At any given temperature, the diffusion of a smaller particle will be more rapid than the larger ones.
What are the Different Types of Diffusion
Since the distribution of molecules occurs in a variety of conditions, diffusion can be classified into two major types:
1) Simple Diffusion
It is the process in which substances move across a biologically active semi-permeable membrane along the concentration gradient without the involvement of any other molecules.
Example: Breathing in oxygen and releasing carbon dioxide out of the body during respiration
2) Facilitated Diffusion
It is the process in which the diffusing material requires the presence of another molecule or a facilitator to perform diffusion.
Example: Glucose, sodium ions, and potassium ions are transported in and out of the cell with the help of specific carrier proteins and protein channels
-
References
Article was last reviewed on Tuesday, November 21, 2023



