Molecule
What is a Molecule
Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. Whenever two or more atoms combine, they form a molecule. It is the smallest unit of a chemical substance having all the properties of that substance. Molecules are neutral and carry no charge. If a molecule splits into smaller pieces, it makes different substances.
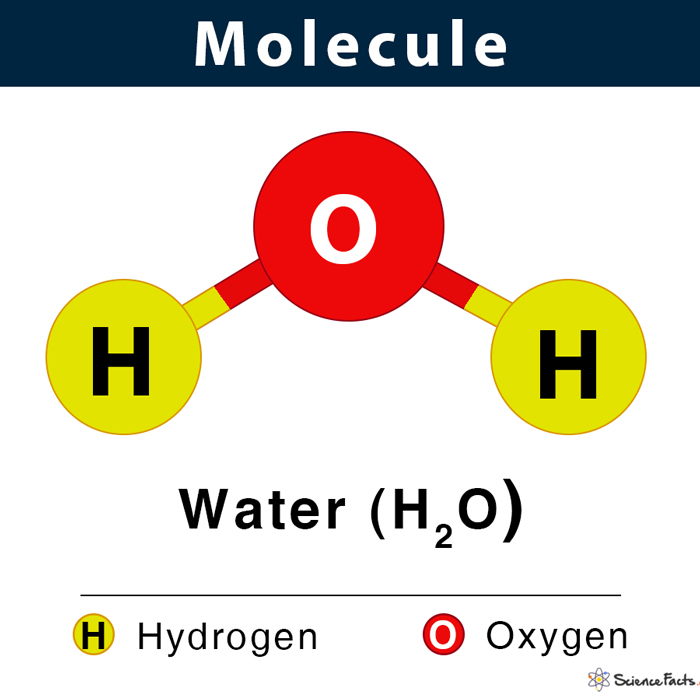
Shown below is the water molecule (H2O).
All objects that we see around us are made of molecules. From living objects such as a plant or an animal, including humans, inanimate objects like a chair, table, wall, door, windows, books, computer, and mobile phones are all made of molecules.
Examples
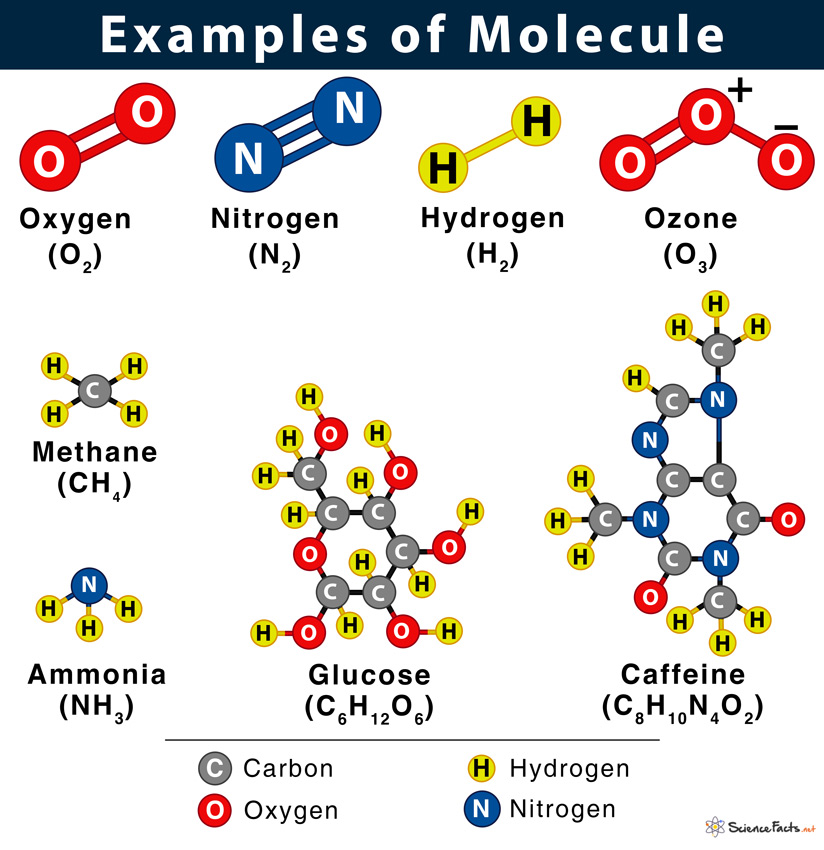
Oxygen (O2), ozone (O3), methane (CH4), sodium chloride (NaCl), and glucose (C6H12O6) are some other common examples of molecules.
Molecules and Elements
Elements are the substance that makes up all matter on earth. Thus, all substances are either made of a pure element or a combination of two or more elements. A molecule is made up of atoms of one or more elements. For example, aluminum is a pure element whose molecule is made up of a single element. Likewise, a molecule of helium consists of a single atom of the element helium. On the other hand, an oxygen molecule is made up of two atoms of the element oxygen.
Molecules and Compounds
Sometimes molecules are made of atoms of two or more different elements. A substance made of those types of molecules is called a chemical compound. Methane is a chemical compound because its molecules contain one carbon atom and four hydrogen atoms.
What is Molecular Formula
Every recipe needs some ingredients. When the ingredients are mixed in a fixed proportion, they make a dish. Chemical substances are made in the same way. About a hundred different atoms are known to us, but we know millions of different substances to exit. How is that possible?
It is possible because each substance is made of different types of atoms, and atoms uniquely combine to form molecules. For example, when two oxygen atoms and one carbon atom combine, they form a carbon dioxide molecule written as CO2. Again, a sugar or a glucose molecule with formula C6H12O6 is made of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Some other examples are ammonia (NH3: one nitrogen atom and three hydrogen atoms), methane (CH4: one carbon atom and four hydrogen atoms), and sucrose (C12H22O11: twelve carbon atoms, twenty-two hydrogen atoms, and eleven oxygen atoms).
Thus, the only way a specific substance is formed is by bonding the precise number and kind of atoms in proper order or orientation.
How Are Molecules Formed
After atoms combine, it is crucial to hold them together for the stability of the molecule. The forces that hold the atoms in a molecule are known as chemical bonds. There are two main types of chemical bonds found in compounds: 1) ionic bonds and 2) covalent bonds. While some compounds have only one type of bond, others possess both of them.
Both types of bonds involve electrons that are negatively charged particles revolving around the nucleus in a fixed path or shell. The shells in an atom follow a specific rule of filling up electrons, known as the octet rule. The outermost shell plays a crucial role in forming a chemical bond. When this shell is partially-filled, the atoms will bond to complete the shell with electrons and form the chemical bond.
1) Ionic Bonds: Occurs when one participating atom gives up an electron to another atom. In other words, one atom receives an electron from another atom to form a stable molecule or compound.
2) Covalent Bonds: Occurs when two or more participating atoms share their electrons between them to form a stable molecule or compound.
Some Fun Facts
- The geometry and arrangement of atoms in a molecule determine the chemical and physical properties of a molecule.
- Molecules have a wide range of shapes like long spiral, pyramidal, spherical, and oval.
- Almost 65% of the human body mass consists of oxygen atoms. The remaining consist of 18% carbon, 9.5% hydrogen, and 3.2% nitrogen atoms.
- Protein, lipids, carbohydrates, and nucleic acids are biological molecules that form our body’s structure.
- DNA, a type of nucleic acid, is a long-coiled molecule with information about the living being.
- A perfect diamond is a molecule made of many carbon atoms arranged in a specific order.
FAQs
Ans. The difference is that an atom is the smallest and the most fundamental unit of matter that can exist independently. A molecule, on the other hand, is made of two or more atoms.
Ans. The difference is that a molecule is a group of two or more atoms held together by chemical bonds. In contrast, a compound is a substance formed by two or more different types of elements combined in a fixed ratio.
-
References
Article was last reviewed on Thursday, February 2, 2023