Heat Engine
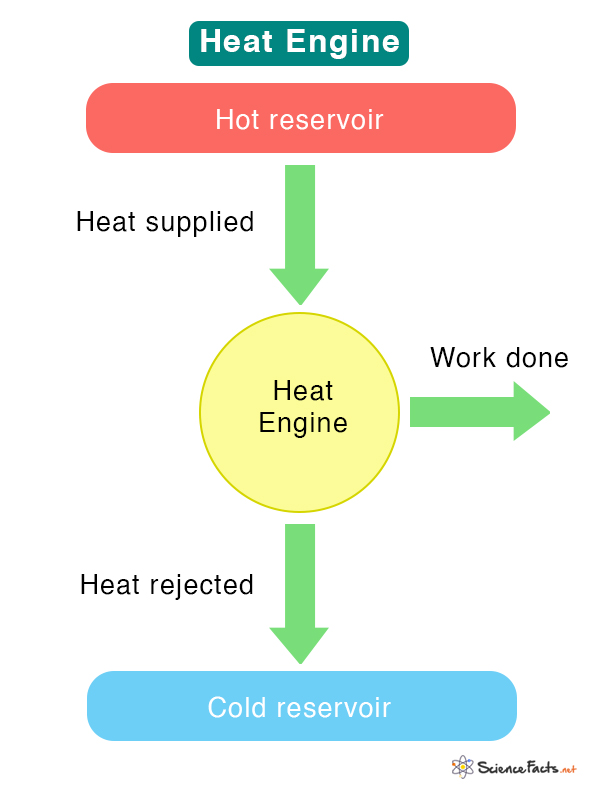
A heat engine is a device that converts heat to mechanical energy and extracts practical work through physical motion. It operates between a hot reservoir that provides heat to a working substance, which is then brought to a lower temperature by dumping the heat into a cold reservoir. The working substance has a finite heat capacity and is usually a liquid or a gas.
An example of a heat engine is an automobile engine. Here, the burning fuel is the hot reservoir, and the surrounding is the cold reservoir in which combustion products are exhausted.
How Does a Heat Engine Work
The hot reservoir is a heat source of infinite heat capacity and is kept at a finite temperature. It provides heat or thermal energy to the working substance. However, not all of the supplied heat gets converted into work. A part of it is rejected into the cold reservoir, called the sink. The rest is utilized to perform the desired work. During its operation, the heat engine passes through a series of thermodynamic processes, thus completing a cycle.
The amount of work extracted from a heat engine depends upon its efficiency. Not all heat engines are 100% efficient, which means that not all heat supplied to the engine is converted into work. Some heat is lost due to friction and wear of mechanical parts, resulting in reduced efficiency.
The first and second laws of thermodynamics establish a relationship between heat and work and limit the operation of a heat engine. The first law is about energy conservation, while the second law limits efficiency and determines the direction of energy flow.
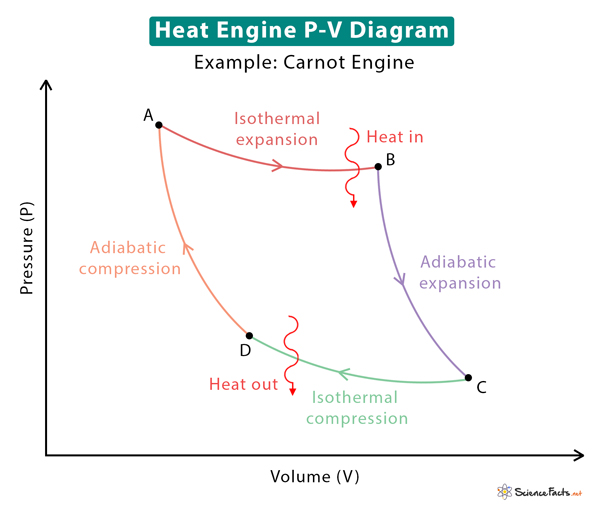
Heat Engine P-V Diagram
The P-V diagram or pressure-volume diagram is a tool to quantify a heat engine. As mentioned before, a heat engine consists of a working substance that can be approximated to an ideal gas. As the engine passes through several thermodynamic processes, the ideal gas law is applied to study the relationships between pressure, volume, and temperature. Thus, the three essential state functions can be tracked through the P-V diagram.
The P-V diagram also gives a visual representation of the work done during volume expansion and the change in internal energy during temperature changes. Recall that the internal energy depends on the temperature. The internal energy changes combined with the first law give the amount of heat absorbed and rejected by the engine.
The image below shows the P-V diagram for the Carnot engine. Various stages of the Carnot cycle are indicated in the diagram.
Efficiency of Heat Engine
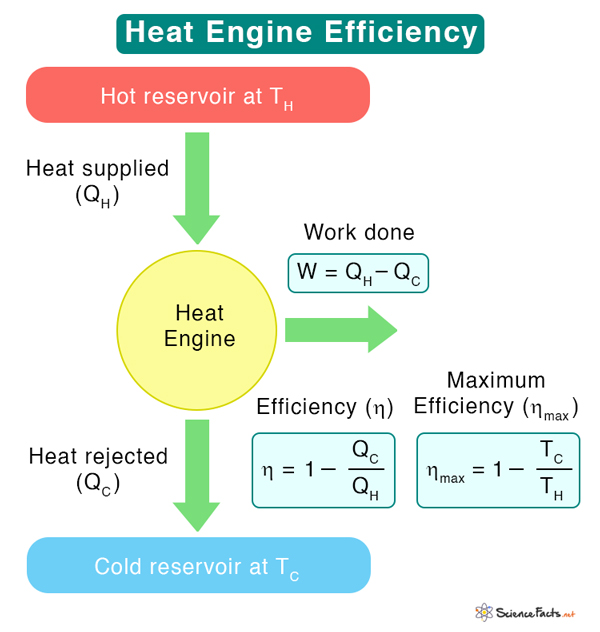
The thermal efficiency of a heat engine determines how much the engine does valuable work for a given amount of heat absorbed from the hot reservoir. It is defined as the ratio of work done to heat input.
Suppose QH is the quantity of heat extracted from the hot reservoir at temperature TH, and QC is the heat dumped into the cold reservoir at temperature TC. Then, the amount of work obtained from the engine is
By definition, efficiency is given by
Since TH and TC are easily measurable, let us express efficiency in terms of temperatures using the second law of thermodynamics and entropy.
Irreversible and Reversible Processes
The change in entropy is
According to the second law, entropy always increases for an irreversible process.
The second law also states that the entropy change is zero for a reversible process.
Which ultimately leads to the expression
However, 1 – TC/TH is the efficiency of a reversible engine such as the Carnot engine.
Therefore, the efficiency of a heat engine is limited by the Carnot theorem. According to this theorem, “Any system working between two given temperatures (hot and cold reservoirs) can never have an efficiency more than the Carnot engine working between the same two reservoirs.”
Types and Examples of Heat Engine
There are two main types of heat engines. They are external combustion engines and internal combustion engines.
External Combustion Engine
In an external combustion engine, the fuel burns outside the engine’s main body, where the action takes place. The steam engine is a typical example of an external combustion engine. Here, coal is burnt to heat water, the working substance. The water is then converted into steam. The steam passes through a cylindrical pipe whose one end consists of a piston moving back and forth. The piston is connected to anything that requires motion, like locomotive wheels. This back-and-forth piston action allows the wheels to rotate and makes the train move forward.
Internal Combustion Engine
In an internal combustion engine, the fuel burns inside the engine. An automobile engine is a perfect example of an internal combustion engine. It consists of several cylinders where the gaseous fuel or gasoline continuously burns, producing heat. Thus, the fuel is the working substance. The heat causes the pistons attached to the cylinders to move back and forth. This mechanical action of the pistons drives the wheels and makes to vehicle move forward.
Internal combustion engines are more efficient than external combustion engines because the fuel is combusted internally, comes in contact with the piston, and fires the engine.
-
References
Article was last reviewed on Thursday, March 23, 2023