Potential Energy Curve
A potential energy surface represents the potential energy of a collection of atoms in terms of their position in a well-defined coordinate system. In one-dimensional systems, the potential energy surface produces a potential energy curve. A potential energy curve depicts the characteristics of a substance. These include composition, bond length, microscopic structure, and microprocessing. These parameters heavily influence the shape of the curve. By studying a potential energy curve, we can clearly understand the properties of a substance.
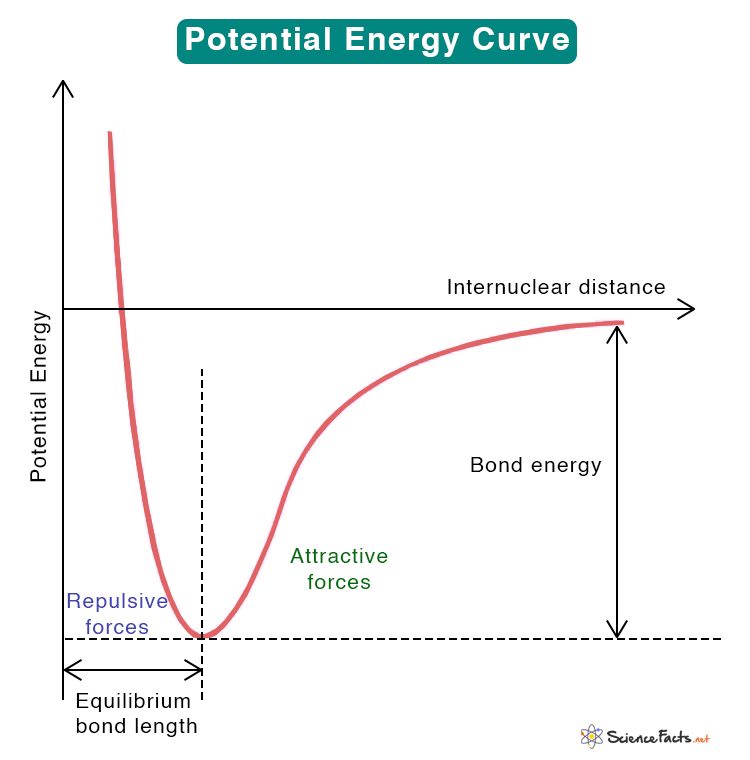
Potential Energy vs. Internuclear Distance Curve
When two atoms are far from each other, there is no interaction between them. It means that the potential energy is zero. As they are brought closer, they experience both attractive and repulsive forces. Attractive forces are due to the attraction between the nucleus and electrons. They are prominent at large distances. Repulsive forces are due to the repulsion between the electrons of one atom with those of the other. They are prominent at short distances.
The curve above shows the energy profile between the two atoms. The vertical axis shows the potential energy, and the horizontal is the internuclear distance, i.e., the separation between the nuclei of the two atoms. The attractive and repulsive forces balance each other where the potential energy is minimum. The internuclear distance at this minimum point is called the equilibrium bond length. Both the equilibrium bond length and the bond energy are depicted in the image.
-
References
Article was last reviewed on Tuesday, January 10, 2023