Chemiosmosis
Chemiosmosis is the process by which hydrogen ions (protons) diffuse to the other side of the biological membrane from high to low concentration. It creates a difference in their concentration (electrochemical gradient) between the two sides of the semi-permeable membrane. The gradient energy synthesizes adenosine triphosphate (ATP) in the cell.
It is the final part of oxidative phosphorylation. The initial part is the electron transport chain.
Where Does Chemiosmosis Occur
In eukaryotes, chemiosmosis occurs in the mitochondria during aerobic cellular respiration and in the chloroplasts during photosynthesis. Since prokaryotes lack these organelles, chemiosmosis occurs in their plasma.
However, chemiosmosis requires a membrane to allow the electrochemical gradient to develop.
Function of Chemiosmosis
Chemiosmosis directly results in the synthesis of ATP. Thus, it is the primary energy source through cellular respiration within the cell. Any defect in the pathway would devoid the cell from producing ATP through oxidative phosphorylation.
Chemiosmotic Theory
Peter D. Mitchell first proposed the chemiosmotic theory in 1961. His theory suggests chemiosmosis is driven by an electrochemical proton gradient across the inner mitochondrial membrane that produces ATP in cells.
How does Chemiosmosis Produce ATP in Cellular Respiration
Before chemiosmosis can start, the electrochemical needs to be established with the help of high-energy molecules, NADH and FADH2. These compounds were formed during glucose oxidation through glycolysis in the cytoplasm to form pyruvate.
The pyruvate is further metabolized to acetyl CoA and then to citric acid or citrate through a series of steps in the citric acid cycle in the mitochondrial matrix. This cycle, with reducing intermediates like NAD and FAD, form NADH and FADH2.
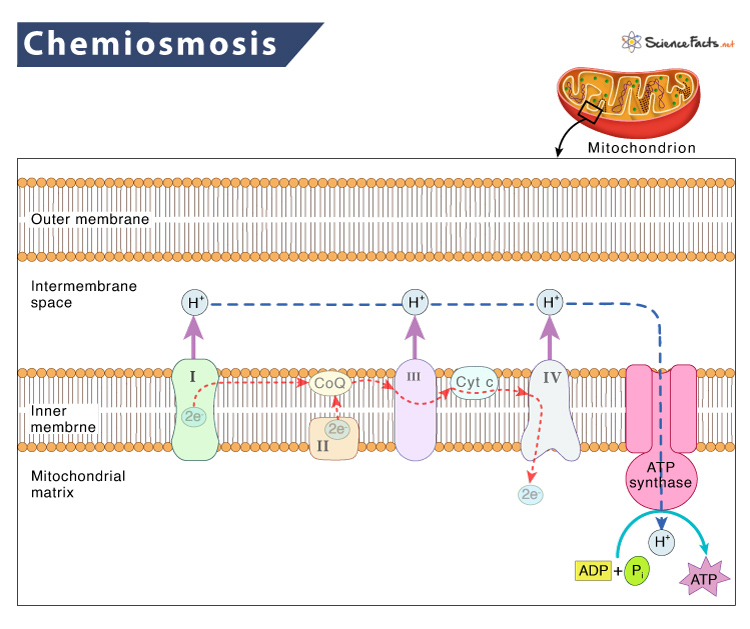
These intermediates act as electron carriers in the electron transport chain. When the electrons move down the electron transport chain, protons are pumped across the mitochondria’s inner membrane, creating an electrochemical gradient, which eventually phosphorylates ADP to ATP. This final step is called chemiosmosis.
Steps of Chemiosmosis
The steps below describe how chemiosmosis occurs in the mitochondria of eukaryotes:
- The sources of electrons in the electron transport chain are high-energy electron carriers like NADH and FADH2. NADH provides electrons to Complex I while FADH2 provides electrons to Complex II
- As electrons move along the electron transport chain cause from a higher to a lower energy level, energy is released. The flow of electrons in ETC is:
Complex I -> Complex II -> Coenzyme Q -> Complex III -> Cytochrome c -> complex IV -> Oxygen
- The hydrogen ions or protons are in lower concentration inside the mitochondrial matrix. The energy liberated by electrons helps to pump protons into the intermembranous space against their concentration gradient. The energy of electrons is stored as an electrochemical gradient
- With the increase in the concentration of protons in the intermembranous space, they move down their concentration gradient along the proton channel in the ATP synthase. This process is coupled to turn the proton ring and liberate energy
- The enzyme ATP synthase uses this energy to phosphorylate ADP, forming ATP on the stroma
How Many ATP Does Chemiosmosis Produce
At the end of aerobic respiration, around 32 ATPs are produced from one glucose molecule. Among these, 2 ATPs are from glycolysis, 4 from the Krebs cycle, and the remaining 28 from chemiosmosis.
Inhibition of Chemiosmosis
Chemiosmosis is inhibited by inhibitors of the electron transport chain or uncouplers. They are protein channels that provide an alternative path for entering the stroma without the involvement of ATP synthase. The energy of the electrochemical gradient gets wasted as heat.
2, 4-dinitrophenol, desaspidin, and dicoumarol are typical examples of uncouplers. The drug aspirin is also an uncoupling agent.
Chemiosmosis in Chloroplasts
In photosynthetic cells of eukaryotes, chemiosmosis occurs in the thylakoid membranes of the chloroplast. It takes place during light-dependent reactions of photosynthesis, where the energy of photons is used to make ATP for dark reactions.
In Non-cyclic Electron Flow
Here, the photoexcited electrons pass through the ETC but do not return to photosystem I.
As photoexcited electrons from the photosystem I move through the electron transport chain, energy is liberated that pumps protons from the stroma of chloroplasts into the lumen of thylakoids. The flow of electrons is:
Photosystem I -> Plastoquinone -> Cytochrome Complex -> Plastocyanin -> Photosystem II -> Ferredoxin -> NADP
The protons move down the concentration gradient back into the stroma, passing through the proton channel of ATP synthase, rotating the ring, and liberating energy, which is used to generate ATP from ADP
In Cyclic Electron Flow
At the end of each cycle, the photoexcited electrons pass through the ETC and return to photosystem I. The flow of electrons is:
Photosystem II -> Ferredoxin -> Cytochrome Complex -> Plastocyanin -> Photosystem II
The mechanism of ATP production is the same as the non-cyclic flow of electrons.
Chemiosmosis in Prokaryotes
Due to the absence of membrane-bound organelles, chemiosmosis in prokaryotes such as bacteria and archaea occurs in the cell membrane. The basic process is similar to mitochondria and chloroplast in eukaryotes.
-
References
Article was last reviewed on Friday, February 3, 2023