Electron Transport Chain
Oxygen is essential to every living species for their survival. Lack of oxygen for an extended period can lead to the death of a living being. Human cells require oxygen in the final stage during aerobic cellular respiration, commonly known as oxidative phosphorylation. Two major components that form oxidative phosphorylation are electron transport chain and chemiosmosis.
What is the Electron Transport Chain
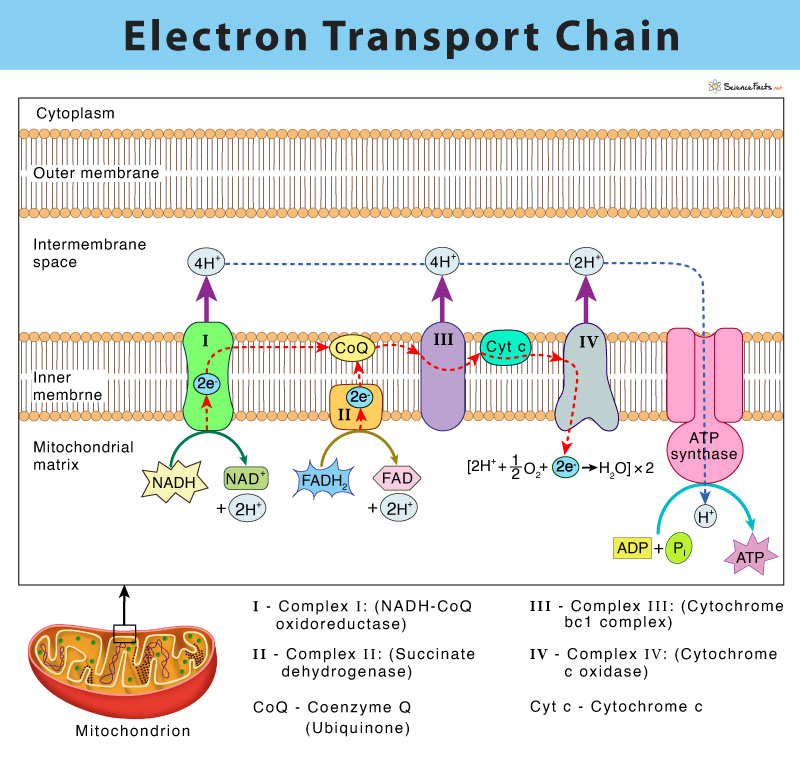
The electron transport chain (ETC) is a group of proteins and organic molecules found in the inner membrane of mitochondria. Each chain member transfers electrons in a series of oxidation-reduction (redox) reactions to form a proton gradient that drives ATP synthesis. The importance of ETC is that it is the primary source of ATP production in the body.
The electron transport chain’s functioning is somewhat analogous to a slinky toy going down a flight of stairs.
Who Discovered the Electron Transport Chain
American biochemist, Albert Lehninger, discovered the electron-transport chain in 1961. The complete ETC was found to have four membrane-bound complexes named complex I, II, III, and IV and two mobile electron carriers, namely coenzyme Q and cytochrome c.
Where does the Electron Transport Chain Take Place
In eukaryotes, multiple copies of electron transport chain components are located in the inner membrane of mitochondria. In bacteria (prokaryotes), they occur in the plasma membrane.
What does the Electron Transport Chain Do
The electron transport chain has two essential functions in the cell:
- Regeneration of electron carriers: Reduced electron carriers NADH and FADH2 pass their electrons to the chain, turning them back into NAD+ and FAD. This function is vital because the oxidized forms are reused in glycolysis and the citric acid cycle (Krebs cycle) during cellular respiration.
- Generating proton gradient: The transport of electron through the chain results in a gradient of a proton across the inner membrane of mitochondria, later used in ATP synthesis.
How does the Mitochondrial Electron Transport Chain Work
The critical steps of the electron transport chain and chemiosmosis are:
- Donation of electrons by electron carriers NADH and FADH2: Two reduced electron carriers NADH and FADH2 produced during earlier stages of cellular respiration transfer their electrons to the specific complex at the start of ETC.
- Transfer of electrons by mobile electron carriers and proton pumping: As electrons flow through the chain, they lose energy, which helps to pump protons (H+ ions) out of the mitochondrial matrix to the intermembrane space. This process creates a proton gradient, also known as the electrochemical gradient.
- Splitting of oxygen to form water: This happens at the end of ETC, where electrons are finally transferred to molecular oxygen, forming a water molecule by accepting H+ ions.
- Synthesis of ATP: As H+ returns to the matrix through the concentration gradient, they pass through a multi-subunit enzyme complex called ATP synthase and result in ATP synthesis.
Steps of Mitochondrial Electron Transport
As discussed above, the entire process of the electron transport chain involves four major membrane proteins that function together in an organized fashion to accomplish ATP synthesis. The events of the electron transport chain are detailed below:
Complex I: (NADH-CoQ oxidoreductase) – Transfer of Electrons from NADH to Coenzyme Q
It is the first complex of the electron transport chain. It is found to be composed of one flavin mononucleotide (FMN) and six-seven iron-sulfur centers (Fe-S) as cofactors.
The process starts by catalyzing the oxidation of NADH to NAD+ by transferring the two electrons to FMN, thus reducing it to FMNH2. Each of the two electrons from FMNH2 is relayed through a series of Fe-S clusters and then to a lipid-soluble carrier molecule known as coenzyme Q (ubiquinone). The reduced QH2 freely diffuses within the membrane.
The above process allows Complex I to pump four protons (H+) from the mitochondrial matrix to the intermembrane space, establishing the proton gradient.
NADH + H+ + CoQ → NAD+ + CoQH2
Complex II: (Succinate dehydrogenase) – Transfer of Electrons from FADH2 to Coenzyme Q
It consists of FAD, and several Fe-S centers.
Complex II is involved in the oxidation of succinate to fumarate, thus catalyzing FAD reduction to FADH2. Next, the electrons from FADH2 reach coenzyme Q through a series of Fe-S centers.
Complex II runs parallel to complex I in the transport chain. However, complex II does not transport protons across the inner mitochondrial membrane, unlike the first complex. Complex II is thus not a part of creating the proton gradient in the ETC.
Succinate + FADH2 + CoQ → Fumarate + FAD+ + CoQH2
Thus, CoQ receives electrons from Complex I and Complex II and gets reduced to CoQH2, which then delivers its electrons to the next complex of the chain, called Complex III.
Complex III (Cytochrome bc1 complex): Transfer of Electrons from CoQH2 to Cytochrome c
It is composed of cytochrome b, c, and a specific Fe-S center, known as cytochrome reductase.
Complex III catalyzes the transfer of two electrons from CoQH2 to cytochrome c. This step results in the translocation of four protons similar to complex I across the inner membrane of mitochondria, thus forming a proton gradient. The reduced CoQH2 is thus oxidized back CoQ while the iron center (Fe3+) in the cytochrome c is reduced to Fe2+.
CoQH2 + 2 cyt c (Fe3+) → CoQ + 2 cyt c (Fe2+) + 4H+
Cytochrome c thus forms the connection between Complex I, II, and III with complex IV with the help of CoQ. Although CoQ carries pairs of electrons, cytochrome c can only accept one at a time.
Complex IV (Cytochrome c oxidase): Transfer of Electrons from Cytochrome c to Oxygen
This step is the last complex of the electron transport chain and comprises two cytochromes a, and a3, which are made of two heme groups and three copper ions.
Complex IV involves transferring two electrons from cytochrome c to molecular oxygen (O2), the final electron acceptor, thus forming water (H2O). The removal of H+ from the system pumps two protons across the membrane, forming a proton gradient.
4 cyt c (Fe2+) + O2 → 4 cyt c (Fe3+) + H2O
Chemiosmosis and Oxidative Phosphorylation
The proton gradient is formed within the mitochondrial matrix, and the intermembrane space is called the proton motive force. Since protons cannot pass directly through the phospholipid bilayer of the plasma membrane, they need the help of a transmembrane protein called ATP synthase to help their cause. Theoretically, ATP synthase is somewhat similar to a turbine in a hydroelectric power plant, which is run by H+ while moving down their concentration gradient. As ATP synthase turns, it catalyzes the addition of phosphate to ADP, thus forming ATP. This process is called chemiosmosis. Chemiosmosis couples the electron transport chain to ATP synthesis and thus complete the oxidative phosphorylation process.
Chemical Equation
6O2 + C6H12O6 + 38 ADP + 39Pi → 38 ATP + 6CO2 + 6H2O
Reactants (Inputs)
- NADH
- FADH2
- O2
End Products (Outputs)
- NAD+
- FAD
- H2O
- ATP
How Many ATP are Generated in the Electron Transport Chain
Roughly, around 30-32 ATP is produced from one molecule of glucose in cellular respiration. However, the number of ATP molecules generated from the breakdown of glucose varies between species. The number of H+ ions that the electron transport chain pumps differ within them.
Where do the 30-32 ATP Count Come From
From a single molecule of glucose producing two ATP molecules in glycolysis and another two in the citric acid cycle, all other ATPs are produced through oxidative phosphorylation. Based on the experiment, it is obtained that four H+ ions flow back through ATP synthase to produce a single molecule of ATP. After moving through the electron transport chain, each NADH yields 2.5 ATP, whereas each FADH2 yields 1.5 ATP.
Given below is a table showing the breakdown of ATP formation from one molecule of glucose through the electron transport chain:
| Name of the Pathway | Net Yield of ATP |
| Glycolysis | 2 ATP (direct) + 3-5 ATP (from 2 NADH) |
| Oxidation of Pyruvate | 5 ATP (from 2 NADH) |
| Citric Acid Cycle | 2 ATP (from 2 GTP), 15 ATP (from 6 NADH) + 3 ATP (from 2 FADH2) |
| Total | 32 ATP |
As given in the table, the ATP yield from NADH made in glycolysis is not precise. The reason is that glycolysis occurs in the cytosol, which needs to cross the mitochondrial membrane to participate in the electron transport chain. Cells with a shuttle system to transfer electrons to the transport chain via FADH2 are found to produce 3 ATP from 2 NADH. In others, the delivery of electrons is done through NADH, where they produce 5 ATP molecules.
Summary of the Process
- Electron Transport Chain is the primary source of ATP production in the body.
- Four protein complexes act as proton pumps that help in the synthesis of ATP.
- Complex I pumps four protons (H+) from the mitochondrial matrix to the intermembrane space and establishes a proton gradient.
- Complex II runs parallel to complex I in the transport chain and delivers its electrons to the next complex chain.
- Complex III moves four protons across the inner membrane of mitochondria and forms a proton gradient.
- Complex IV reduces the oxygen (O2) molecule by adding two protons to form a water molecule (H2O).
- Proton motive force enables hydrogen ions (H+) diffuse back into the matrix via transmembrane enzyme ATP synthase, thereby creating ATP from ADP.
NADH + H+ → Complex I → CoQ → Complex III → Cytochrome c → Complex IV → H2O
↑
Complex II
↑
Succinate
Inhibitors of Electron Transport Chain
The following are considered to be inhibitors of the electron transport chain:
- Rotenone
- Antimycin A
- Cyanide
- Carbon Monoxide
Electron Transport Chain in Bacteria
The electron transport chain in bacteria is much more complicated compared to the electron transport chain in eukaryotes. The reason is that multiple electron donors and electron acceptors are participating in the process. The entire process is similar to eukaryotes. It is carried out by four membrane-bound protein complexes (Complex I, II, III, and IV) and two mobile electron carriers, cytochrome and quinine.
Electrons can enter the chain at three different levels: a) at dehydrogenase, b) at the quinone pool, or c) at the cytochrome level. The electrons entering the chain flows through the four complexes with the help of the mobile electron carriers and are finally transferred to an oxygen molecule (for aerobic or facultative anaerobes) or other terminal electron acceptors such as nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules (for anaerobes).
-
References
Article was last reviewed on Thursday, February 2, 2023