Diffusion vs Osmosis
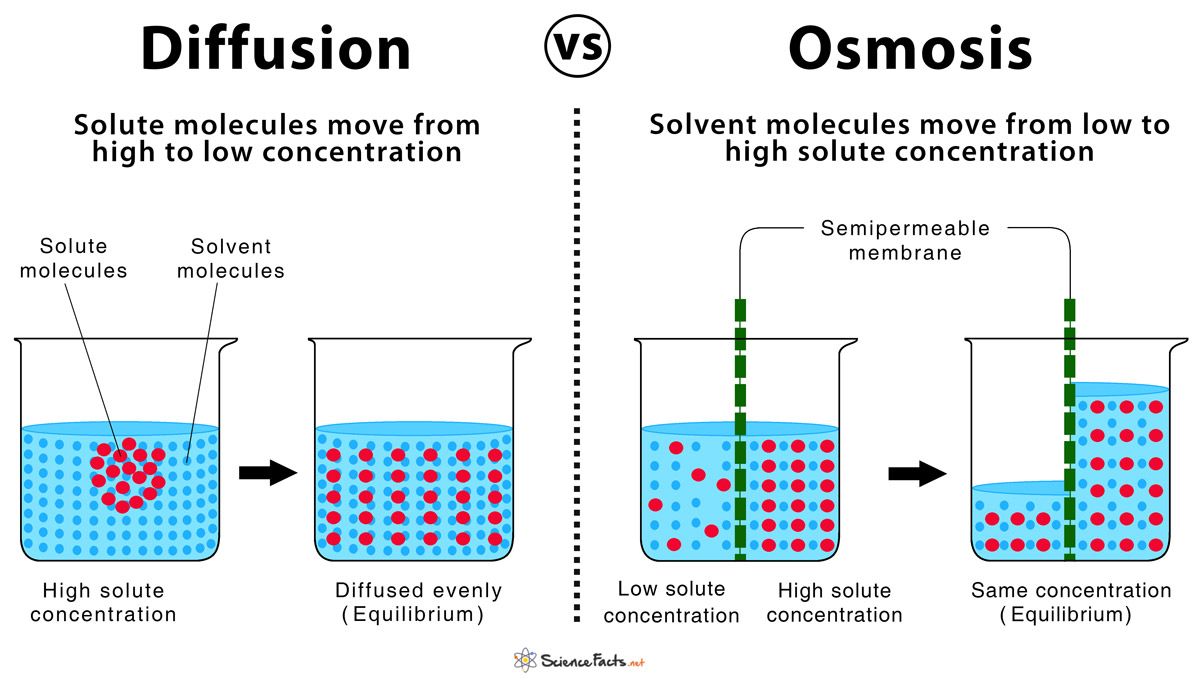
Diffusion and osmosis are both important passive transport methods where molecules move to equalize the concentration of the region undergoing the process, to attain equilibrium. Although both the processes share many common properties, they are contrasting in many aspects.
| Basis For Comparison | Diffusion | Osmosis |
| The Process | The movement of solutes existing in any state of solid, liquid or gas from a region of their higher concentration to the region of lower concentration, but without a semipermeable membrane | The movement of solvent especially water from the region of high solute concentration to the region of low solute concentration, through a semipermeable membrane |
| Medium | Takes place in any medium of solid, liquid, and gas | Takes place only in the liquid medium |
| Material | Applied to all states of solid, liquid, and gas | Applied only for the solvent part of the solution |
| Properties of Molecules | Depends on the size and electric charge of molecules | Depends on the particle concentration |
| Concentration Gradient | Moves along the concentration gradient | Moves down the concentration gradient |
| Semipermeable membrane (cell membrane) | The motion is direct and does not always require the semipermeable membrane or cell membrane | The motion is always through the semipermeable membrane or cell membrane |
| Rate of the process | Fast process | Slow process |
| Free energy | Only depends on the free energy of the substance | Depends on the rate of reduction of the free energy of one solvent |
| Example | The scent of perfume filling a whole room within seconds | Plant root hairs taking up water from the soil |
| Purpose and Significance in living organisms | 1. Important in animals for producing energy for the cell, during respiration it helps in the exchange of gases
2. In plants, it helps in transpiration and photosynthesis |
1. Important in animals for maintaining the water potential of the cell, transporting nutrients, and in cell-cell diffusion
2. In plants, it maintains turgidity, provides mechanical support, prevents excess water loss, and helps in the absorption of water from the soil |
How are Diffusion and Osmosis Alike
- Neither process can initiate unless there is a difference in the concentration of particles between two regions.
- The processes are complete only when the concentration of the particles becomes even in both regions (equilibrium).
- Both are spontaneous transport processes, which means they do not require any input of extra energy to occur
- In both, particles move from an area of higher concentration to lower concentration
- Internal factors like solute potential and water potential do not affect either process
References
-
References
Article was last reviewed on Saturday, July 4, 2020




I love how your post simplifies the reverse osmosis process for non-science readers (like myself!).
When a ro system boasts a water-to-waste ratio, is the wasted water due to the osmosis or the diffusion process?
Reverse osmosis works by reversing the principle of osmosis.
Thank you