Osmosis
What Is Osmosis
Osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute concentration through a semipermeable membrane in order to equalize their concentrations on both sides of the membrane.
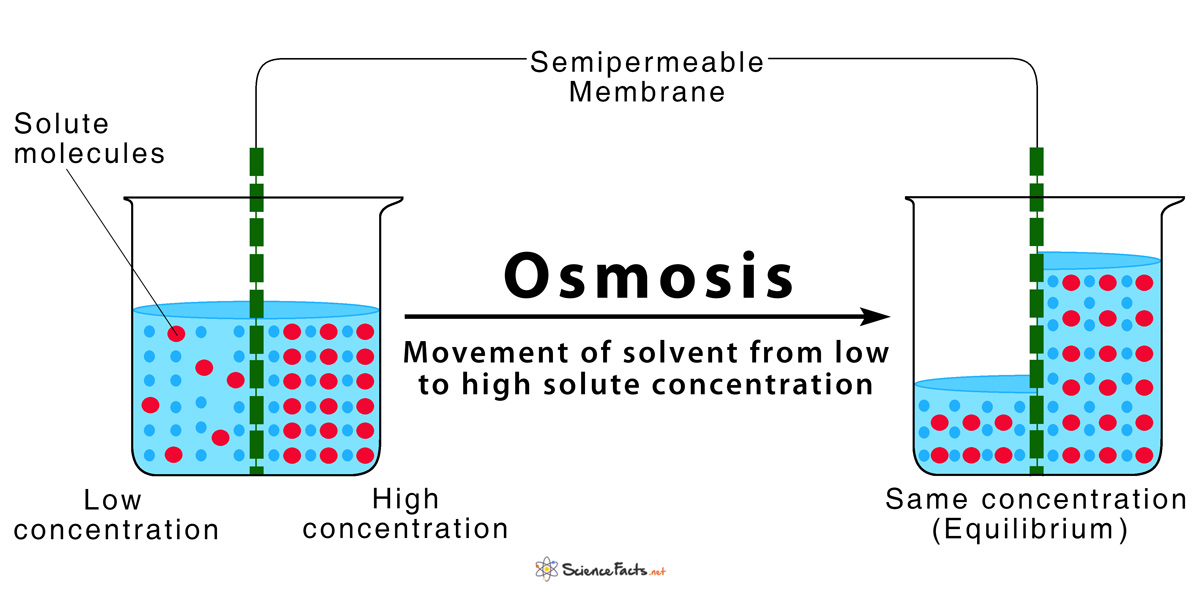
What Causes Osmosis and Why does it Occur
When two solutions of different concentration are separated by a semipermeable membrane, the water molecules tend to move from the region of low solute concentration (high water potential) towards high solute concentration (low water potential), to equalize their concentration on both sides of the membrane or attain a state of equilibrium.
Osmosis was first thoroughly studied in 1877, by German plant physiologist Wilhelm Pfeffer. The general term ‘osmose’ (now osmosis) was introduced in 1854 by British chemist Thomas Graham.
Basic Characteristics of Osmosis
- Requires a semipermeable membrane
- A slow and spontaneous process
- Occurs in liquid medium
- Requires no energy expenditure and thus also called passive diffusion
- Movement of water occurs from a region of high water potential to a region of low water potential
- The process continues until the concentration of the solution becomes even on both the sides of the semipermeable membrane
Examples of Osmosis
It is an important phenomenon occurring in both plants and animals. Some everyday-life examples of osmosis are:
In Plants
- Roots of plants have a higher solute concentration than the surrounding soil, so water flows into the roots which are absorbed by the plants through osmosis
- The opening and closing of guard cells, responsible for gas exchange in plants, depends on the absorption of water by osmosis
In Animals
- Human body infected with cholera-causing bacteria perform osmosis to reverse the flow of water absorption in the intestine, causing excess water loss, leading to severe dehydration and sometimes death
- If a freshwater fish is transferred to saltwater or vice versa, the fish will die of too much osmosis, which will disrupt the balance of salts in its body.
- Cells take up nutrients and minerals into the cell and also get rid of their waste products by osmosis
What Factors Affect Osmosis
1) Temperature – Warmer the temperature, higher is the rate of osmosis.
2) Area of Interaction – More the surface area of interacting molecules, higher is the rate of osmosis.
3) Concentration Gradient or Osmotic Gradient – Greater the difference in concentration gradient between the regions, higher is the rate of diffusion
4) Osmotic Pressure – Higher the hydrostatic pressure exerted by a solution across a semipermeable membrane from a pure solvent, slower is the rate of diffusion
Different Types Of Osmotic Solutions and Their Effect on Osmosis
The relative concentration of solutes present in the solution is called its tonicity. The tonicity determines the rate and direction of osmosis. Osmotic solutions are classified into the following types:
a) Isotonic Solutions: Have the same concentration of solutes both inside and outside the cell. Here there is no net movement of solvents across the membrane as the amount entering and leaving out the cell are equal.
b) Hypotonic Solutions: Have a higher concentration of solutes inside the cell than outside. When this occurs, more solvent enters the cell compared to the amount that leaves out, to balance the concentration of solute on both sides of the membrane.
c) Hypertonic Solutions: Have a higher concentration of solutes outside the cell than inside it. Here more solvent leaves the cell compared to the amount that enters inside the membrane.
What Are The Different Types Of Osmosis
Based on the direction of water movement, osmosis is classified into two main types:
Endosmosis
It is the process by which water moves inside the cell when placed in a hypotonic solution causing them to swell up and become rigid. Endosmosis occurs because the solute concentration of the surrounding solution is less compared to the concentration inside the cell.
Exosmosis
It is the process by which water moves out the cell when placed in a hypertonic solution causing them to become flaccid. Exosmosis occurs because the solute concentration of the surrounding solution is higher than inside the cytoplasm.
In some extreme cases of exosmosis, the cells lose excess water, and the cell membrane separates from the cell wall, a process known as plasmolysis.
-
References
Article was last reviewed on Friday, February 17, 2023



