Lactic Acid Fermentation
Lactic acid fermentation is anaerobic respiration occurring without oxygen that breaks down sugars, producing energy in the form of ATP. This process is so named because lactic acid is a byproduct of this reaction.
A typical example of lactic acid fermentation is making yogurt by the bacteria Lactobacillus.
History of Lactic Acid Fermentation
- It was first discovered in 1780 by a Swedish chemist, Carl Wilhelm Scheele, who isolated lactic acid from sour milk as impure brown syrup.
- In 1789, Lavoisier named this milk component ‘acide lactique’, later called lactic acid.
- In 1857, Pasteur discovered lactic acid as a fermentation metabolite generated by specific microorganisms.
- In 1881 supporting Pasteur’s discovery, French scientist Frémy produced lactic acid, which gave rise to the first industrial production of lactic acid in the United States.
Where and When Does It Occur
It occurs in some bacteria and specific animal cells without oxygen.
In Bacteria
Bacteria that carry out lactic acid fermentation are commonly referred to as Lactic Acid Bacteria (LABs). This process is a survival strategy in those bacteria as it produces the energy required for their metabolism and helps them reduce the pH of their environment through the synthesis of lactic acid. Such an acidic condition makes the environment unsuitable for other microorganisms, thus reducing their growth competition.
While some bacteria are strictly anaerobic, some can switch between aerobic and anaerobic-based respiration depending on oxygen availability.
In Animal Cells
When muscle cells have insufficient oxygen supply during vigorous exercises, cells undergo anaerobic respiration to meet their energy demand. Through this anaerobic respiration, lactic acid, a byproduct, accumulates in the muscles, causing weakness and a burning sensation.
Besides muscle cells, lactic acid fermentation also occurs in red blood cells (RBCs) as they lack mitochondria to produce energy aerobically.
Process of Lactic Acid Fermentation
The process of lactic acid fermentation occurs in two steps:
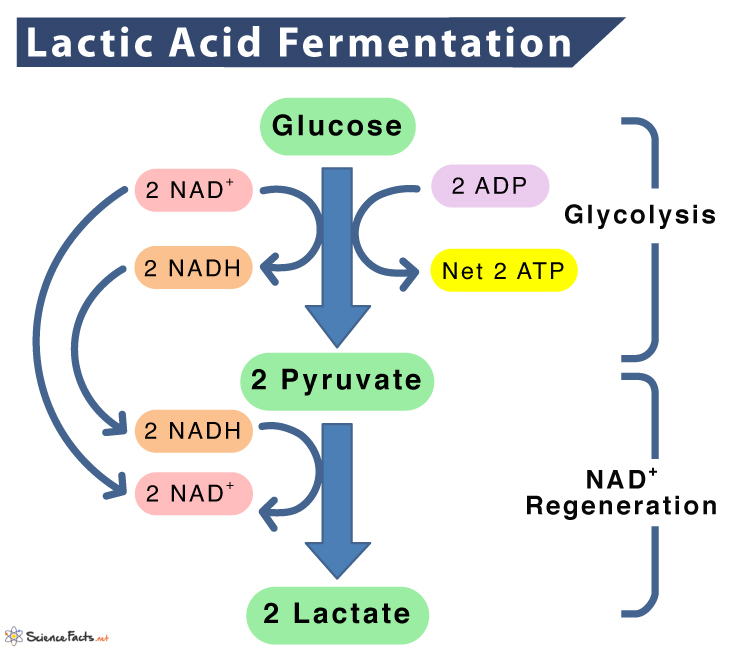
Step 1: Glycolysis
It involves the conversion of glucose to 2 molecules of pyruvic acid, creating 2 ATP and 2 NADH. Glycolysis occurs in the absence of oxygen. NADH acts as the electron carrier, and pyruvate is the final electron acceptor in lactic acid fermentation.
Glucose + 2 ATP + 2 NAD+ -> 2 Pyruvic acid + 2 NADH + 2 ATP……. (1)
Step 2: Conversion of Pyruvic Acid to Lactic Acid
Each pyruvic acid molecule is converted to lactic acid producing 4NAD+ as the byproduct. 2 of the NAD+ go back to continue glycolysis. This reaction occurs in the presence of the enzyme lactate dehydrogenase.
2 Pyruvic acid + 4 NADH -> 2 C3H6O3 (Lactic Acid) + 4 NAD+ ……. (2)
From equations (1) and (2), we get the lactic acid formation equation:
Glucose + ADP + NADH -> C3H6O3 (Lactic Acid) + ATP + NAD+
This lactic acid fermentation pathway where glucose is first broken down to pyruvate by glycolysis, then the glucose to pyruvate producing 2 ATPs is called homolactic acid fermentation.
This pathway is commonly employed by some Lactobacilli, Pediococcus, and Streptococcus species.
Other Lactic Acid Fermentations
There are two other forms of lactic acid fermentation. Each is performed by a particular species of lactic acid bacteria producing different byproducts.
Heterolactic Acid Fermentation is similar to homolactic fermentation. However, instead of 2 lactic acid molecules, only one molecule of lactic acid is synthesized, along with a molecule each of ethanol and carbon dioxide. The equation of heterolactic acid fermentation is:
Glucose + ADP -> 2 C3H6O3 (Lactic Acid) + C2H5OH + Ethanol + CO2 + ATP
Bifidium Pathway, or the Bifidobacterium shunt pathway, produces acetate and lactate as byproducts and is produced by Bifidobacteria. The equation is:
2 Glucose + 5 ADP -> 3 Acetate + 2 C3H6O3 (Lactic Acid) + 5 ATP
Uses of Lactic Acid Fermentation
As we now know, humans need to perform lactic acid fermentation when our body needs much energy in a hurry. It also serves as an alternative method of generating energy in muscle cells under stress due to strenuous exercises.
Also, lactic acid fermentation helps certain bacteria such as Lactobacilli carry out respiration even without oxygen. Thus, lactic acid fermentation serves as a rescue pathway in bacteria, animals, and humans.
This type of fermentation has a high commercial use. It is widely used in the industry to produce fermented food products and beverages, for example, yogurt, cheese, fermented pickles (such as kimchi, sauerkraut), soy sauce, and sourdough bread.
Lactic Acid Fermentation vs. Alcoholic Fermentation
Similarity
- Occur in the absence of oxygen
- Glycolysis is the first step
Differences
Common differences between lactic acid and alcoholic fermentation are shown in the following table.
| Basis | Lactic Acid Fermentation | Alcoholic Fermentation |
|---|---|---|
| 1. Process | A biological process where glucose is converted to lactate | A biological process where glucose is converted to ethanol and carbon dioxide |
| 2. Occurs in | Occurs in Lactobacillus spp, yeasts, and animal cells such as muscle cells and RBCs | Occurs in yeasts and certain bacteria |
| 3. Byproducts | Lactate | Ethanol and carbon dioxide |
| 4. Enzymes Involved | Lactate dehydrogenase and pyruvate decarboxylase | Alcohol dehydrogenase and pyruvate decarboxylase |
| 5. Uses | Produces yogurt, cheese, fermented pickles, and sourdough bread, among others | Produces vinegar, wine, and beer, among others |
-
References
Article was last reviewed on Wednesday, August 31, 2022



