Topoisomerase
Topoisomerases, or DNA topoisomerases, are enzymes that create single or double-stranded nicks in DNA during its replication or transcription to relieve the stress in a supercoiled DNA molecule. Thus, topoisomerases change the topology of the DNA strand.
The first DNA topoisomerase was discovered by James Wang in 1971. It was initially named omega protein and is now called Escherichia coli topoisomerase I.
Structurally, topoisomerases have a catalytic core involved in the cleavage and rejoining DNA strands, a DNA-binding domain, and an optional ATP binding site found in some enzymes.
How Does Topoisomerase Work
Topological stress arises due to the double-stranded nature of the DNA strand. Sometimes, DNA coils upon itself more tightly than its usual nature (DNA supercoiling). It is necessary because DNA is a long molecule and must fit inside the tiny nucleus of our cells. However, as DNA is copied and manipulated during various cellular processes, it becomes more twisted.
In such situations, topoisomerase acts like a molecular scissor and untangler. It carefully cuts and reseals the DNA strands, relieving the excess tension and preventing DNA from becoming a tangled mess. It also provides the enzymes involved in processes like replication, transcription, chromosome segregation, and recombination to have access to the DNA.
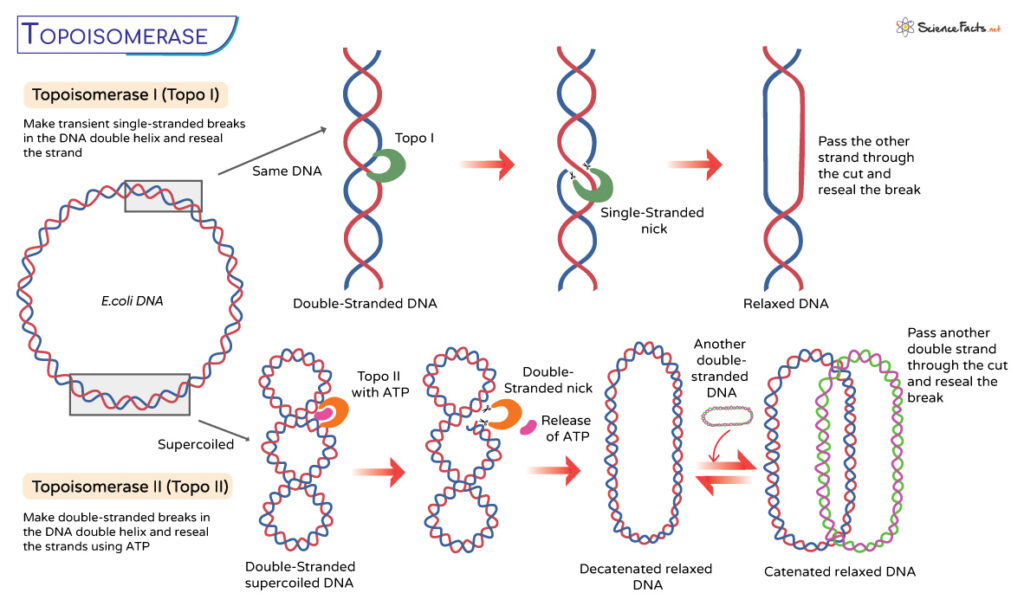
Types of Topoisomerases
Topoisomerases transiently break one or both DNA strands, classifying the enzymes into 2 types: Type I topoisomerase and Type II topoisomerase.
Type I Topoisomerases
These monomeric enzymes make transient single-stranded breaks in the DNA double helix. It functions independently of ATP and changes the linking number by 1. They perform DNA supercoil relaxation, unknotting of single-strands circles, and decatenation.
Type I topoisomerases are of 3 types:
- Type IA topoisomerase introduces a single-strand break by forming a tyrosyl-phosphate bond between a tyrosine residue in the enzyme and a 5′-phosphate in the DNA. The region of DNA where the break occurs is called the gate or the G-segment. This cleavage allows the passage of another DNA segment (strand passage) through it, followed by resealing of the G-segment. Examples include prokaryotic topo I and III, eukaryotic topo IIIα and IIIβ, and archaeal reverse gyrase.
- Type IB topoisomerase makes a transient single-strand break in the DNA by forming a tyrosyl-phosphate bond between a tyrosine in the enzyme and a 3′-phosphate in the DNA. Instead of the strand-passage process, type IB enzymes initiate a swivel or rotation of the cleaved strand around the intact strand. Examples include eukaryotic nuclear and mitochondrial topo I and viral topo I.
- Type IC topoisomerase is structurally distinct but follows a similar function mechanism to the Type IB enzymes. Topo V, found in Methanopyrus kandleri, is the only member of this group.
Type II Topoisomerases
Type II topoisomerases are homodimeric or heterotetrameric enzymes that introduce double-stranded breaks in the DNA molecule. It is an ATP-dependent enzyme that changes the linking number by 1.
The process starts when type II enzymes attach to the double-stranded DNA substrates. It then proceeds through a strand-passage mechanism. While all type II topoisomerases are involved in DNA relaxation, DNA supercoiling, unknotting, and decatenation, an archetypal bacterial topoisomerase, DNA gyrase, introduces negative supercoiling.
Type II topoisomerases are of 2 types:
- Type IIA topoisomerase catalyzes transient double-stranded breaks in DNA by forming tyrosyl-phosphate bonds between tyrosines in the enzyme and 5′-phosphates in the bases of the opposite strands. Bacterial gyrase, topo IV, and eukaryotic topo IIα and topo IIβ are examples of topoisomerase.
- Type IIB topoisomerase also induces transient double-stranded breaks in DNA by forming tyrosyl-phosphate bonds between a tyrosine residue in the enzyme and 5′-phosphates in opposite strands of the DNA. However, here, the double-stranded breaks have a 2-base stagger. Initially found in Achaea, they are also found in plants such as topo VI and topo VIII.
Topoisomerases function to unwind the DNA double helix by making transient cuts in the DNA. Thus, they are like surgeons, acting as molecular scissors that reduce tension in DNA and then repair it during replication.
Functions of Topoisomerase
DNA can become overwound (positive supercoiling) or underwound (negative supercoiling) during replication and transcription. Topoisomerases (type I and II) adjust this supercoiling and relieve this torsional stress, preventing DNA strands from tangling and breaking. Sometimes, DNA forms complex structures like knots and tangles during various cellular processes. Topoisomerase, especially type II B enzymes, resolves these structures, prevents DNA damage, and ensures stability.
These roles of topoisomerases allow cells to carry out some of the critical biological processes.
DNA Replication
For DNA replication to proceed uninterrupted, the double helix must unwind so that the DNA polymerase can synthesize new complementary strands. Type I topoisomerases help relax the supercoiled regions ahead of the replication fork, thus ensuring smooth and accurate replication.
DNA Repair
When DNA sustains damage, such as single-stranded breaks or lesions, topoisomerases can assist in rejoining the broken ends. They also repair DNA damage caused by radiation and chemicals.
Transcription
RNA polymerase needs access to the DNA template to synthesize RNA molecules during transcription. Supercoiled DNA hinders this process. Topoisomerases help by relaxing the DNA, making it accessible for transcription. They relive the torsional stress caused by the movement of RNA polymerase along the template DNA.
Cell Division
During cell division, topoisomerase II plays a vital role in condensation and segregation of chromosomes. They help correctly distribute the parent chromosome to the two daughter cells, preventing errors.
Gene Expression
Topoisomerase indirectly influences gene expression by changing the accessibility of DNA to transcription factors and some other regulatory proteins.
Topoisomerase vs. Helicase
Both topoisomerase and helicase help in the unwinding of the DNA. However, they are different in many ways:
| Basis | Topoisomerase | Helicase |
|---|---|---|
| 1. Main Function | Separates double-stranded DNA, single-stranded RNA, or a DNA-RNA hybrid | Makes single or double-stranded nick in the DNA to remove negative or positive supercoiling |
| 2. Types | Topoisomerase I and Topoisomerase II | DNA helicases and RNA helicases |
| 3. Act on | Only DNA | Both DNA and RNA |
| 4. Energy Use | Topoisomerase I does not utilize ATP, while Topoisomerase II uses ATP for its function | Utilizes ATP for its function |
Topoisomerase Inhibitors
Some chemicals inhibit the activity of the topoisomerases by blocking the ligation step, causing cell death by apoptosis. Some common topoisomerase inhibitors are listed below:
Topoisomerase type I Inhibitors
- Irinotecan
- Topotecan
- Camptothecin
Topoisomerase II Inhibitors
- Etoposide
- Doxorubicin
- Epirubicin
-
References
Article was last reviewed on Saturday, November 25, 2023