Wave-Particle Duality
Wave-particle duality is a fundamental concept in physics that describes the dual nature of matter and light. It suggests that particles like electrons and photons can exhibit wave-like and particle-like properties depending on the experimental setup. This concept revolutionized our understanding of the microscopic world and led to the development of quantum mechanics.
According to the wave-particle duality concept, particles can be described by wavefunctions, which are mathematical functions that represent the probability distribution of finding a particle at different locations. These wavefunctions capture the wave-like behavior of particles.
Theories Explaining Wave-Particle Duality
Several theories have been proposed to explain this intriguing phenomenon. Three important ones are the de Broglie hypothesis, Heisenberg uncertainty principle, and Schrödinger’s wave equation.
1. De Broglie Hypothesis
Proposed by Louis de Broglie in 1924, this hypothesis suggests that all particles, including matter, have wave-like properties. De Broglie proposed that the wavelength of a particle is inversely proportional to its momentum. It means that even macroscopic objects, like baseballs or cars, have an associated wavelength. However, it is tiny and difficult to observe. This idea paved the way for the development of wave-particle duality.
De Broglie proposed that a particle of momentum p would have a wavelength λ given by the equation:
λ = h/p
Where h is the Planck’s constant. This wavelength is known as de Broglie wavelength.
2. Heisenberg Uncertainty Principle
Formulated by Werner Heisenberg in 1927, the uncertainty principle asserts that it is impossible to simultaneously determine the precise position and momentum of a particle with absolute certainty. This principle places a fundamental limit on our ability to measure these properties accurately. It implies that particles do not possess definite positions or momenta until observed, suggesting a wave-like behavior where probability distributions describe the particle’s properties.
The uncertainty principle can be conceptualized in mathematical notation, which states that the product of the uncertainties in position (Δx) and momentum (Δp) cannot be less than Planck’s constant over four pi.
(Δx) (Δp) ≥ h/4π
3. Schrödinger’s Wave Equation
Developed by Erwin Schrödinger in 1926, this equation describes how the wave function of a quantum system evolves over time. The wave function represents the probability distribution of finding a particle at a given position and time. It incorporates wave-like and particle-like characteristics, allowing us to calculate the possible outcomes of experiments involving quantum systems. Schrödinger’s equation provides a powerful tool for understanding and predicting the behavior of particles on a quantum level.
Examples Demonstrating Wave-Particle Duality
Having explored the theories explaining wave-particle duality, let us explore real-world examples demonstrating this intriguing phenomenon and understanding the fascinating duality of matter. Here are three well-known examples.
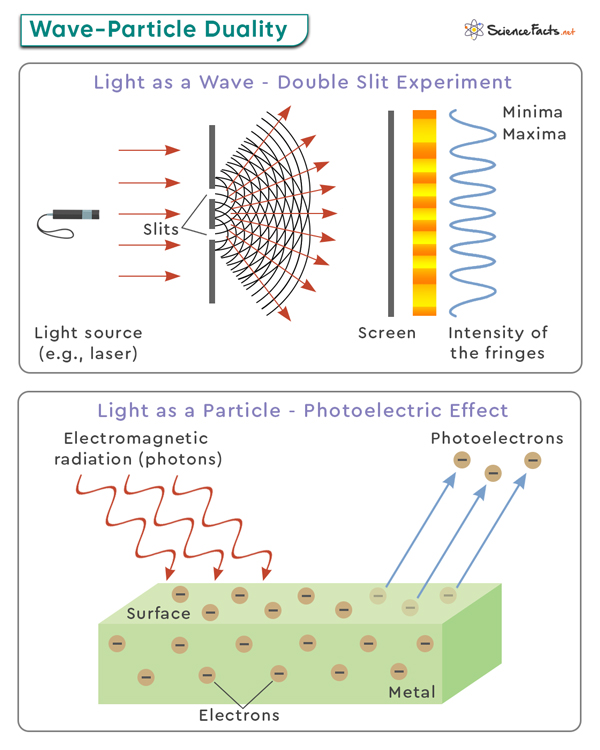
1. Young’s Double-slit Experiment
In this experiment, a beam of light is sent through two closely spaced slits and observed on a screen behind them. When the light passes through the slits, it creates an interference pattern on the screen, similar to what would be expected from waves. However, when the experiment is repeated with individual particles, such as electrons or even larger molecules, they also create an interference pattern, suggesting that these particles can behave like waves.
2. Photoelectric Effect
The photoelectric effect occurs when light shines on a metal surface and causes the emission of electrons. According to classical wave theory, light intensity should determine the energy of emitted electrons. However, experiments revealed that only light above a specific frequency (threshold frequency) causes electron emission, regardless of intensity. This observation concluded that light can act as discrete packets of energy called photons, exhibiting particle-like behavior.
We can analyze the photoelectric effect by using the energy conservation law. The total energy of the incoming photon must be equal to the kinetic energy of the ejected electron plus the energy required to eject the electron from the metal, which is described mathematically by the photoelectric equation:
E = φ + KE
Where:
E represents the energy carried by a single photon of light of frequency ν (E = hν).
KE represents an electron’s kinetic energy after being emitted from the material.
φ is the work function and refers to the minimum energy required to remove an electron from a specific material.
3. Davisson-Germer Experiment
In this experiment, a beam of electrons was directed at a crystal surface and observed for scattering patterns. The scattered electrons were expected to behave like particles bouncing off the crystal lattice. Surprisingly, the results showed a diffraction pattern similar to what is expected from waves passing through a double slit. This experiment demonstrated that electrons can exhibit wave-like behavior, supporting the idea of wave-particle duality.
These examples highlight how various experiments have shown that particles like photons and electrons can exhibit wave-like and particle-like properties depending on their interactions. Wave-particle duality challenges our intuitive understanding of matter, which is now a central concept in modern physics.
-
References
Article was last reviewed on Wednesday, November 13, 2024








I am interested in a way of estimating the effect of a boat of certain mass and speed, travelling straight on course, through still water, and causing a wave of water, travelling outwards from the bow, at an angle of 45 degrees. Observation shows the wave will continue for a certain distance, seemingly depending on the weight/displacement, of the hull, the speed of travel of the boat, and water temperature, assuming no ambient wind effects, and water level being at an elevation of 600 feet above sea level. Now what are the other factors determining how far the wave will travel until it is extinguished?? How are there other factors to be considered in order to predict by equation, how far the wave would continue before it is extinguished by the inherent friction of water molecules, gravitational effects, and whatever additional forces are acting on the generated wave?
The distance a wave generated by a boat travels before being extinguished is influenced by factors beyond the boat’s displacement, speed, and water temperature. Key additional factors include water depth, wave frequency and wavelength, water viscosity, surface tension, salinity, wave interactions, bottom friction, obstacles, and air resistance.
Has the importance of temporal location also been considered as an explanation of wave – particle duality.
In fact, could it be that the particle we see/locate in space is simply that which we can only locate in our slice of spacetime and it is not so much a case of everywhere, but also everywhen? In other words the particle is moving so fast that it only appears to be in many places at once (wave) – creating a wave like effect. But it is simply the same particle. ie a spacetime object but only capable of being perceived and measured as a point in a linear slice of spacetime. Yes, it can interfere with itself because of a superfast, seemingly instantaneous, temporal effect. But the wave does not really exist. The duality is not actual. It just seems that way.
Why are light and matter both both particles and waves
While sound is only a wave?
Perhaps sound is also both particle and wave.
Also, if we are waves then do we also create a Doppler effect on each other?
If we are waves and all our senses have developed to detect other waves that are coming toward us rather than moving away from us then
Light and matter exhibit wave-particle duality, meaning they behave as both particles and waves, as shown by quantum mechanics. Sound, however, is purely a mechanical wave and doesn’t have the same dual nature because it arises from macroscopic vibrations of particles in a medium. While humans, like all matter, have quantum wavefunctions, the effects of wave behavior, such as the Doppler effect, are negligible on our scale. Our senses have evolved to detect various waves, like light and sound, but primarily those that are moving toward us rather than away.