Why Does Salt Melt Ice
You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. It helps to melt the ice by lowering its freezing point, a phenomenon known as ‘freezing point depression’. Let’s go into the details of how this physical change happens.

How Does Table Salt Melt Ice Faster
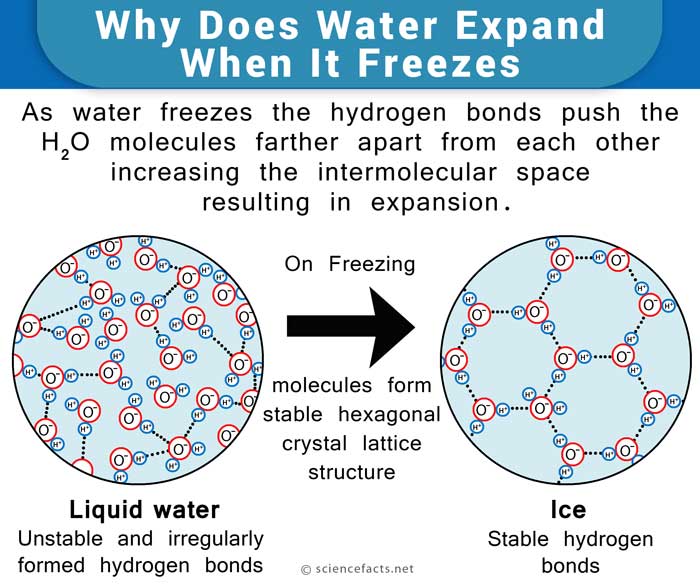
As we know, 0oC or 32oF is the freezing point of water, meaning it freezes into ice at that temperature. However, not all the water converts into ice at once. Some of the water molecules attach themselves to the ice and freeze, while some of the ice molecules attach themselves to the liquid water and melt. This inter-conversion rate is constant so that we have as many water molecules turning into ice as we have ice molecules turning into water.Salt needs at least a little water to be able to lower the freezing point, and as mentioned, ice at its freezing point is always surrounded by a layer of water. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. However, the ice continues to convert to water. This causes more and more water to be formed, as even though the freezing rate has halted, the melting rate remains the same.
What Happens When Salt Is Added To Ice – Explaining At the Molecular Level
Salt or sodium chloride is an ionic compound. When it is put on the surface of the ice on roads, it mixes with the water (a polar compound) on the surface and breaks into its elements, sodium (Na+) and chloride (Cl–) ions. This would not have been possible if there was no water along with the ice. Now, these ions occupy the space in between the liquid water molecules and prevent them from coming close together to form solid ice. This lowers the freezing point of the water.
The salt in the solution also comes in the way of the rigid structure of the ice it is in contact with, weakens its bonds and causes it to melt. More water dissolves the salt further, breaking into more and more ions, continuing the melting. So ice begins to melt before the water can freeze.
When Does Regular Salt Stop Melting Ice
Normally, a 10% solution of regular table salt can lower the freezing point to 21oF (-6oC). This means as long as the temperature is above 21oF the ice will melt in a 10% salt water solution. But if the temperature of the surroundings falls below that level, you would need more salt to lower the freezing point further. However, adding any amount of table salt does not affect the ice in extreme cold weathers when the temperature falls below 0oF (-18oC).
Sometimes other salts, such as calcium chloride, potassium chloride, etc. are also spread on snow to make it melt. It is because they can separate into more ions. More ions in water would mean more obstruction to the crystal structure of ice, hence more melting. These salts can lower the freezing point of water further than rock salt.
Apart from salts, other substances such as sugar and alcohol can produce the same effect, as anything disrupting the normal structure of water and ice would prevent it from freezing. But less abundance and a higher cost restrict their use as de-icing agents.
FAQs
1. Will Epsom salt melt ice?
– Epsom salt does melt ice, but it takes longer than table salt.
2. Which type of salt melts ice the fastest?
– Though a variety of commercial ice melting products is used nowadays that contain complex combinations of salts and act very fast, of the common salts, calcium chloride is known to melt fastest, even at temperatures as low as -25oF (-32oC).
Article was last reviewed on Tuesday, December 10, 2019