Atomic Nucleus
What is the Atomic Nucleus
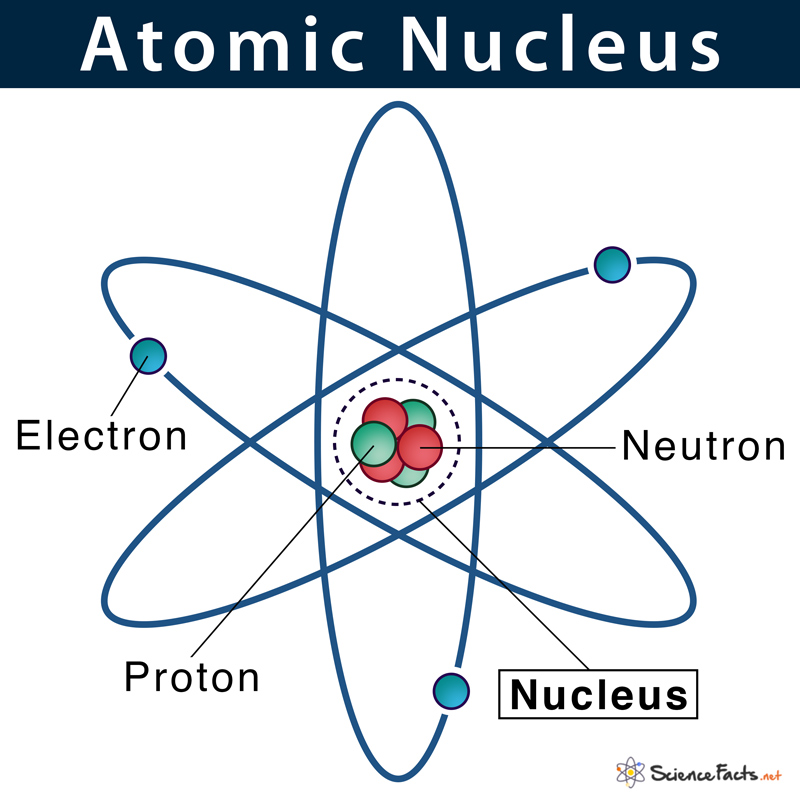
The nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. A nucleus accounts for more than 99.9% of an atom’s mass but is 100,000 times smaller than it in size. They are thus the densest part of an atom.
The word ‘nucleus’ means ‘kernel of a nut’. In 1844, Michael Faraday used the nucleus to describe the ‘central point of an atom’. Ernest Rutherford described the exact meaning of atoms in 1912.
Who Discovered the Nucleus?
Ernest Rutherford, in 1911 was credited with the discovery of the nucleus based on the 1909 Geiger–Marsden gold foil experiments, which includes a series of landmark experiments.
Its Size
The nucleus is tiny when compared to the size of an atom. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms, such as uranium). This size amounts to only 1/100,000 times the radius of the whole atom. If we consider an atom to that of a football stadium, the nucleus would be about the size of a pea.
What are Nucleus Made of
As mentioned, protons and neutrons are the two types of subatomic particles found tightly packed within the nucleus. The space surrounding the nucleus is mostly empty spaces where a cloud of negatively charged electrons is revolving at fixed distances from the center.
Charge of the Nucleus
An atomic nucleus is made of only protons and neutrons. Since protons carry a positive charge (+1) and neutrons are electrically neutral, the nucleus, on the whole, has a net positive charge.
Mass of Nucleus
Electrons being a fundamental particle it has a legible mass. Proton and neutron, on the other hand, have considerable mass. Thus, the sum of the mass of proton and neutron determines the mass of the atom. In other words, the nucleus contains virtually all the mass of an atom.
Regular units of mass, such as gram (g) or Kilogram (Kg), cannot weigh anything as small as an atom. Thus, scientists have created a new unit of mass. It is called the Atomic Mass Unit (u). Carbon-12 is taken as its reference, and one amu is equal to 1/12th the weight of one atom of Carbon 12.
1 amu = 1.660539 10-24 g
What Makes Nucleus Stable
We know, particles with opposite charges attract each other while particles of the same charge repel. This means positively charged protons in the nucleus should push away from each other, thus breaking the nucleus apart. Then why the nucleus does not break apart? This stability is achieved because an even stronger force called nuclear force holds protons and neutrons together in the nucleus by overcoming the proton-proton repulsive force.
FAQs
Ans. The energy is stored in the nucleus of an atom is called nuclear energy.
-
References
Article was last reviewed on Thursday, February 2, 2023






thank you