Neutron
What is a Neutron
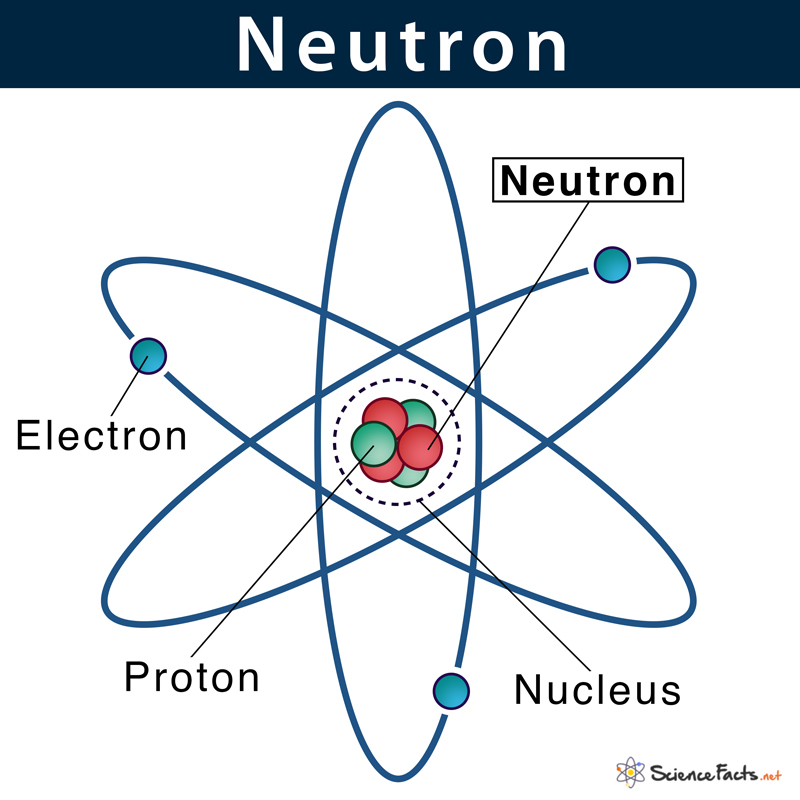
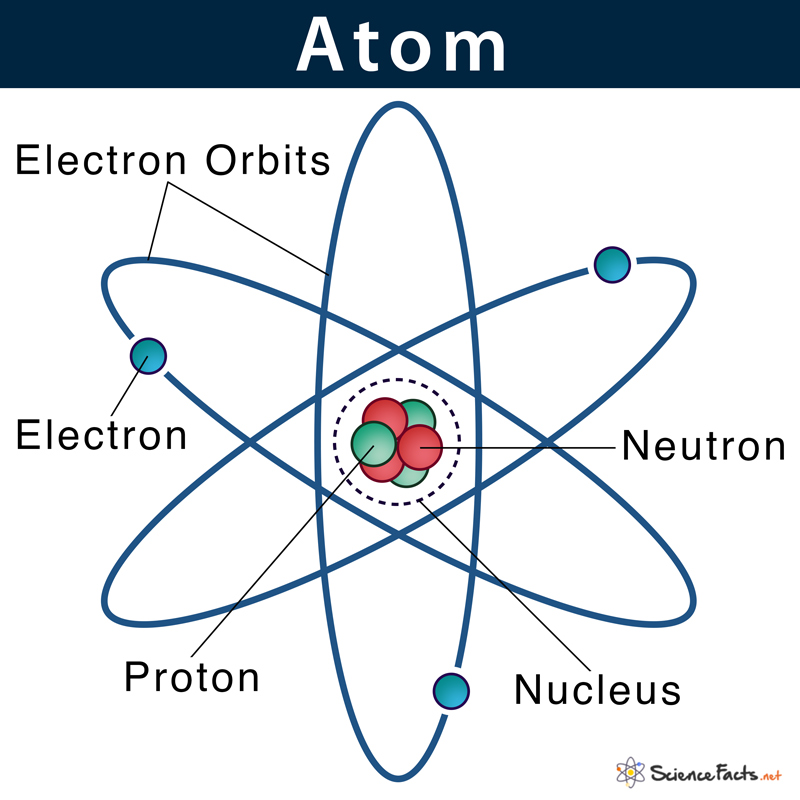
A neutron is an uncharged sub-atomic particle found in all atomic nuclei and has a mass similar to a proton. A neutron is thus one of the three particles that constitute an atom. The other two being proton and electron. The common symbol of the neutron is n or n0. Neutrons are used in nuclear reactors where it helps to continue the process of nuclear chain reaction.
The word ‘neutron’ was derived from the Latin word ‘neuter’, meaning neither, and the Greek suffix ‘on’ is consistent with the names of all sub-atomic particles, including proton and electron.
Who Discovered Neutron
While discovering proton in 1917, Ernest Rutherford found evidence of the existence of a neutrally charged within the atomic nucleus that could solve the disparity between the atomic number of an atom and its atomic mass. Later in 1932, James Chadwick, a student of Rutherford, discovered the neutron during his experiment when he bombarded beryllium atom with high energy alpha particles.
Where are they Located
Neutrons, similar to protons, are found inside the atom’s nucleus, a tiny dense region at the center of the atom.
What are Neutrons Made of
Neutrons consist of two types of fundamental particles known as quarks and gluons. Each neutron contains three quarks where two of them are called down quarks, and the third is called an up quark. Gluons carry a strong nuclear force that holds quarks together. Quarks were discovered by Murray Gell-Mann and George Zweig in 1964, while Gluonswere discovered by John Ellis and his fellow workers in 1979.
Characteristics
1. Size: Not being a fundamental particle, it has a measurable size. A neutron has a diameter of 1.7×10−15 m, which is almost similar to a proton.
2. Charge: Unlike proton and electron, the neutron has no charge. In other words, they are electrically neutral and thus are represented as n0. Here, ‘0’ stands for zero charges.
3. Mass: It has a mass equal to one atomic mass unit (u) or 1.675 x 10-24 g. This is slightly greater than the mass of a proton and roughly 1,836 times the mass of an electron. Together with protons, they make up virtually all of the mass of an atom.
4. Number in Atoms: Generally, all atoms have the same number of neutrons as protons within the nucleus. For example, the number of neutrons in the element Osmium is 114.
5. Movement: They travel in straight lines and produce shadow of the objects placed in their path.
6. Other: The number of neutrons may vary for the atoms of the same element. Atoms of the same element that vary in their numbers of neutrons are called isotopes. For example, 99% of carbon atoms have six neutrons, while the rest 1% have seven or eight neutrons.
-
References
Article was last reviewed on Friday, February 3, 2023