Band Theory
Band theory is a key concept in solid-state physics that helps us understand how electrons move in different materials. It explains why some materials are good conductors of electricity while others are insulators or semiconductors.
Band theory explains how electrons are distributed across different energy levels, or bands, within a solid. These energy bands form because of the interactions between many atoms in a crystal lattice. The way electrons behave within these bands determines if a material can conduct electricity.
Energy Band Diagram
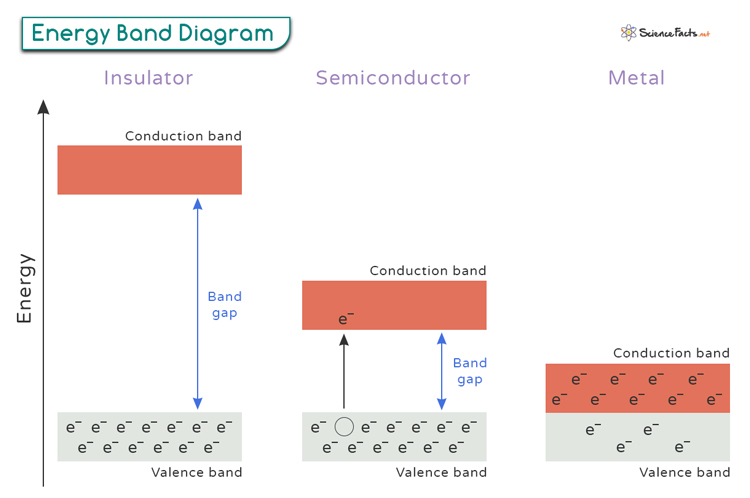
Understanding the energy band diagram is crucial for learning about the electrical characteristics of materials. An energy band diagram shows the energy levels that electrons can have within a material. The vertical axis represents the energy levels, and the horizontal axis shows the positions of electrons in the crystal structure of the material. The two main energy levels we focus on are the valence band and the conduction band.
Valence Band
The valence band is the energy level in an atom that holds the outermost electrons. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds.
At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. Electrons in the valence band have lower energy than those in the conduction band, so they do not usually help conduct electricity. These electrons are tightly bound to their atoms and cannot move freely.
However, when the material is given extra energy from heat or light, some electrons in the valence band can gain enough energy to jump to the conduction band. When they do this, they can move freely and contribute to the material’s electrical conductivity or affect its optical properties.
Conduction Band
The conduction band is a range of energy levels in a material that sits above the valence band. Normally, at room temperature, the conduction band contains energy levels that do not have electrons in them. However, when an external energy source, such as heat or an electric field, is applied to the material, electrons can gain enough energy to move from the valence band to the conduction band. This process frees the electrons from their individual atoms, allowing them to move freely and conduct electricity.
The mobility of electrons in the conduction band is essential for a material’s ability to conduct electricity. When an electric field is applied, these free electrons can move in response to the field, creating an electric current. A material’s ability to conduct electricity depends on two main factors: the width of the gap between the conduction band and the valence band (called the energy band gap) and the number of available electrons in the conduction band.
Energy Band Gap
The energy band diagrams for metals, insulators, and semiconductors provide a clear visual representation of their different electrical properties.
Conductors
Conductors have overlapping valence and conduction bands, allowing electrons to move easily through the material and conduct electricity. Examples of conductors include copper (Cu), aluminum (Al), and silver (Ag).
Insulators
Insulators have a large band gap between their valence and conduction bands, making it difficult for electrons to move across them. Examples of insulating materials include glass, rubber, plastic, ceramics, and wood.
Semiconductors
Semiconductors fall somewhere in between, with a small band gap that can be overcome by external factors like temperature or doping. Common examples of semiconductors include silicon (Si), germanium (Ge), gallium arsenide (GaAs), and indium phosphide (InP).
Doping is the process of adding small amounts of other elements (called dopants) to a semiconductor to change its electrical properties.
- N-type semiconductors are doped with elements like phosphorus, which add extra electrons and facilitate electron movement.
- P-type semiconductors are doped with elements like boron, which create “holes” in the valence band. These holes can move through the material and act like positive charges.
-
References
Article was last reviewed on Tuesday, July 23, 2024