Crystal Structure
A crystal is comprised of matter arranged in a structured three-dimensional pattern of atoms, molecules, or ions. A crystal structure is a distinctive arrangement of atoms, molecules, or ions in a crystal. It is highly ordered and repetitive, creating a characteristic pattern that defines the crystal’s shape and properties.
The Role of Unit Cell in Crystal Structure
A fundamental idea in crystal structures is the unit cell. It is the smallest unit of volume that allows identical cells to be arranged together to occupy all available space. The geometrical basis of all crystals is the lattice. A lattice can be viewed as a regular and infinite arrangement of points or atoms, where each point or atom has an identical surrounding environment. This concept applies equally in one, two, and three-dimensional spaces. The entire crystal lattice can be constructed by repeatedly reproducing the pattern of the unit cell in all directions.
A crystal structure focuses on how the atoms are arranged inside the crystal. However, crystallographers are interested in the overall shape and size of the crystal as well as its external characteristics. To accomplish this, they need to know about the crystal system.
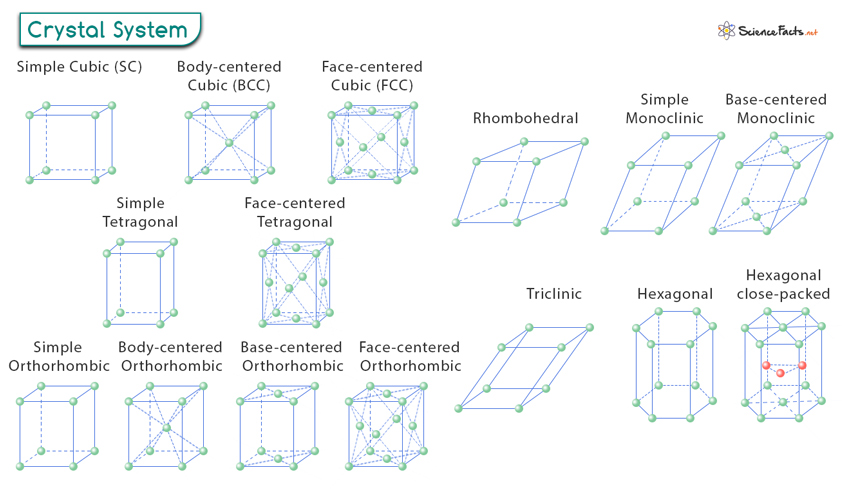
Crystal System
A crystal system refers to the classification of crystals based on the geometric arrangement and symmetry of their lattice structures. There are seven main crystal systems:
- Cubic (isometric) – Characterized by three equal-length axes that intersect at right angles.
- Tetragonal – Characterized by three mutually perpendicular axes, two of which are equal in length, while the third is longer or shorter and perpendicular to the other two.
- Orthorhombic – Characterized by three mutually perpendicular axes, all of different lengths.
- Trigonal (rhombohedral) – Characterized by three equal axes that intersect at equal angles, not necessarily right angles.
- Monoclinic – Characterized by three unequal axes, two of which intersect at an oblique angle, while the third is perpendicular to the plane of the other two.
- Triclinic – Characterized by three unequal axes that intersect at oblique angles.
- Hexagonal – Characterized by four axes, three of which are equal in length and intersect at 120 degrees, with a fourth axis perpendicular to the other three.
Example of Crystal Structure
Sodium Chloride (NaCl)
The crystal structure of sodium chloride (NaCl), commonly known as table salt, is a prime example of a cubic crystal lattice. In this arrangement, the sodium (Na+) and chloride (Cl–) ions are positioned in an alternating pattern, forming a three-dimensional grid-like structure.
The sodium chloride crystal lattice is characterized by the close packing of the positive sodium ions and negative chloride ions. Each sodium ion is surrounded by six chloride ions, and each chloride ion is surrounded by six sodium ions, creating a highly stable and symmetrical structure.
This crystal structure is responsible for the unique physical and chemical properties of sodium chloride, including its high melting point, electrical conductivity, and ability to form ionic bonds easily.
FAQs
Ans. Yes, all minerals have a well-defined crystal structure.
Ans. The crystal structure of a mineral is determined by the conditions under which it forms, including its chemical composition, temperature, pressure, and the presence of other substances.
Ans. Most liquids freeze by the formation of crystalline solids from the uniform liquid.
Ans. The primary difference between atomic structure and crystal structure lies in their definitions: atomic structure refers to how atoms are organized within a single molecule or a cluster of molecules, whereas crystal structure concerns the specific arrangement of atoms within a solid substance.
-
References
Article was last reviewed on Friday, June 7, 2024