Diamagnetism
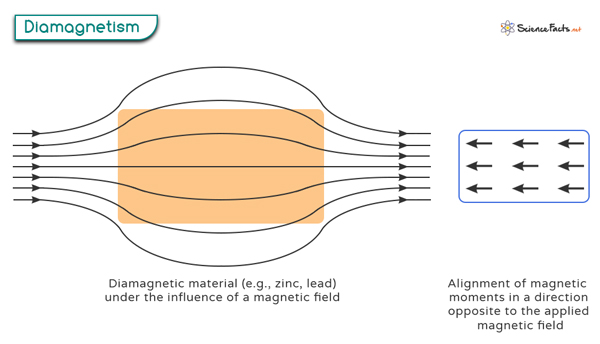
Diamagnetism is a property exhibited by certain materials, known as diamagnetic materials, that cause them to create a weak magnetic field in the opposite direction when placed in an external magnetic field. It results in the material being repelled by the magnetic field rather than attracted to it, as with paramagnetic and ferromagnetic materials.
What Causes Diamagnetism
Diamagnetism is a property exhibited by certain atoms, elements, and molecules due to the orbital motion of electrons. The electron configuration of the atom or molecule plays a crucial role in determining its diamagnetic properties. The arrangement of electrons within the atomic or molecular orbitals directly affects its magnetic behavior.
Diamagnetism occurs when all the electrons in an atom are paired up in the orbitals. When subjected to an applied magnetic field, the electrons realign their orbital motion so that their magnetic moments are opposed to the external field. As a result, a weak repulsive force between the diamagnetic atoms and the external magnetic field is generated. It is important to note that diamagnetism is inherent to all atoms. However, it becomes more noticeable when there are no unpaired electrons present.
Diamagnetic Materials
Here are some examples of diatomic elements and molecules:
Diamagnetic Elements
- Bismuth (Bi) is the most diamagnetic element. Its electron configuration, [Xe] 6s2 4f14 5d10 6p3, shows that it has several paired electrons up to the 5d orbital and only three unpaired electrons in its 6p orbital. Therefore, its diamagnetism outweighs its paramagnetic tendencies.
- Copper (Cu) is another example of a diamagnetic element. Its electron configuration, [Ar] 4s1 3d10, shows all electrons paired in the 3d orbital, leaving one unpaired electron in the 4s orbital. The presence of paired electrons in the 3d orbital outweighs the paramagnetic effect of the unpaired electron in the 4s orbital, leading to an overall diamagnetic behavior in copper.
Diamagnetic Molecules
- The molecular orbital diagram of water (H2O) shows that all eight electrons in the molecular orbital are fully paired, resulting in a net electron spin of zero.
- Like water, molecular orbital theory predicts that nitrogen (N2) gas is also diamagnetic. Each nitrogen atom has three unpaired electrons, but in the N2 molecule, all electrons are paired.
- Fluorine (F2) gas is a combination of two fluorine atoms. Each fluorine atom has one unpaired electron. However, in the F2 molecule, there are no unpaired electrons, making it diamagnetic.
Applications
Diamagnetic Levitation
Diamagnetic levitation is an interesting phenomenon in which strong magnetic fields repel diamagnetic materials that appear to defy gravity. In maglev trains, powerful superconducting magnets are used to create a strong magnetic field, and the train itself is equipped with diamagnetic materials, typically in the form of magnets or coatings. These materials actively repel the magnetic field generated by the tracks, causing the train to levitate above them. This virtually frictionless mode of transportation reduces energy consumption. It allows for significantly higher speeds than traditional rail systems, thus showcasing the intriguing interplay between magnetism and gravity.
Materials Characterization
Another critical application of diamagnetic materials is in material characterization and quality control. Diamagnetic susceptibility measurements can be used to assess the purity and composition of substances. Scientists and engineers can gain insights into the material’s molecular structure and integrity by subjecting a sample to a known magnetic field and measuring its diamagnetic response. This technique finds utility in various industries, from pharmaceuticals and chemistry to mineral exploration and gemology.
-
References
Article was last reviewed on Friday, September 15, 2023