Paramagnetism
Paramagnetism is an exciting phenomenon that refers to the magnetic behavior of certain materials, known as paramagnetic materials, that are feebly attracted to an external magnetic field (magnet). Unlike ferromagnetic materials, paramagnetic materials only display a temporary magnetization that disappears once the external field is removed.
What Causes Paramagnetism
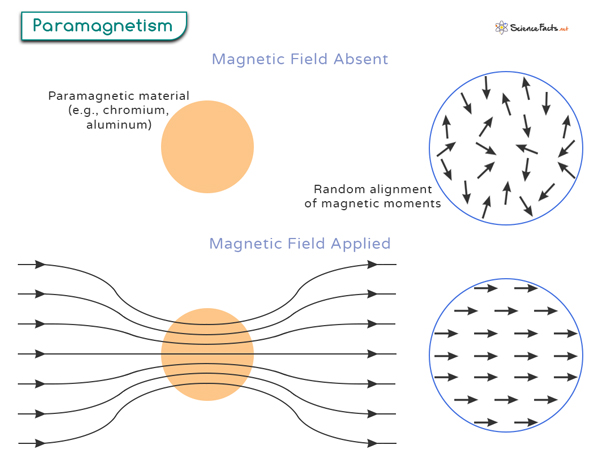
Paramagnetism is caused by the presence of unpaired electrons in the atomic or molecular orbitals of a material. When materials have unpaired electrons, these electrons possess intrinsic magnetic moments due to their spin and orbital angular momentum. These magnetic moments can interact with an external magnetic field, resulting in an alignment of their spins in the field direction, as shown in the image.
Paramagnetic Materials
Here are some examples of paramagnetic elements and molecules.
Elements
- Aluminum (Al) is paramagnetic because it has unpaired electrons in its atomic structure, specifically in the 3p orbitals.
- Titanium (Ti) exhibits paramagnetism because it has two unpaired electrons in the 3d orbital.
- Gadolinium (Gd) is a well-known paramagnetic material. It has eight unpaired electrons in its electronic configuration.
- Chromium (Cr) is paramagnetic due to its six unpaired electrons in the 4s and 3d orbitals.
Molecules
- Oxygen (O2) gas is paramagnetic because molecular orbital theory predicts it contains two unpaired electrons in the two π* orbitals, attracting liquid oxygen to a magnetic field.
- Nitrogen dioxide (NO2) molecule is paramagnetic because it has an odd number of electrons, resulting in unpaired electrons in its molecular orbitals.
Magnetic Susceptibility of Paramagnetic Materials
Paramagnetic materials exhibit a positive magnetic susceptibility, which attracts them to a magnetic field. The degree of attraction depends on the strength of the external magnetic field and the material’s magnetic susceptibility.
Magnetic susceptibility depends upon the temperature according to Curie’s law. The law states that the magnetic susceptibility of a material is inversely proportional to its absolute temperature (T). In equation form, this relationship can be expressed as:
Here, C is called the Curie constant, a characteristic property of the material that depends on the strength of the atom and the density of magnetic moments.
Applications
With its unique magnetic properties, paramagnetism finds various applications across diverse scientific fields and industries. Its ability to respond to external magnetic fields and provide valuable insights into the electronic structure of materials makes it a valuable tool in various areas. Here, we explore some critical applications of paramagnetism.
- Magnetic Resonance Imaging (MRI): Medical imaging is one of the most well-known applications. Paramagnetic agents, such as gadolinium-based contrast agents, are used in MRI scans to enhance the contrast of images, creating detailed and high-resolution images of soft tissues, organs, and blood vessels.
- Magnetic Susceptibility Measurements: Paramagnetic materials are often used in laboratory settings to measure the magnetic susceptibility of substances. This property is used to study the electronic structure and bonding of materials, including minerals, superconductors, and magnetic materials.
- Magnetic Resonance Spectroscopy: Beyond medical imaging, paramagnetism is crucial in magnetic resonance spectroscopy techniques such as electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR) spectroscopy. These methods are used in chemistry, physics, and biochemistry to study the electronic and structural properties of molecules, investigate chemical reactions, and analyze biomolecular structures.
In conclusion, paramagnetism’s diverse applications underscore its significance in modern science and technology. Paramagnetic properties continue to drive numerous innovations and discoveries across various disciplines.
-
References
Article was last reviewed on Friday, September 29, 2023








Can paramagnetic elements protect us from radiation
No, paramagnetic elements cannot protect us from radiation. Magnetic fields can only deflect charged particles.
How can one notify a paramagnetic element. More like examples of such element
I am assuming you mean determine. It can be determined by examining its electron configuration: if it shows unpaired electrons, then the substance is paramagnetic.