Hypertonic Solution
What is a Hypertonic Solution
A solution having a higher solute concentration or lower water content than another solution is known as a hypertonic solution (Latin ‘hyper’ means ‘over’ or ‘above’).
Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap.
Seawater, sugar syrup, corn syrup are some common examples of hypertonic solutions.
What it Does in a Cell
Generally as the cell interior has higher solute concentration (hypertonic), it means that the extracellular medium is low in solutes (hypotonic). This difference in concentration allows passive diffusion of several ions, nutrients and water in and out of the cell. As a result, water moves out of the hypertonic cell following its concentration gradient. This phenomenon helps to maintain the solute and water concentration in both intracellular and extracellular medium. Specialized protein channels, called aquaporins, and some transporter molecules present in the cell membrane aid in this process.
Thus, an equal osmotic pressure exists in both the cell and its surroundings. By this spontaneous homeostatic process, the cell maintains its rigidity and solute concentration.
What Happens to a Cell When Placed in a Hypertonic Solution
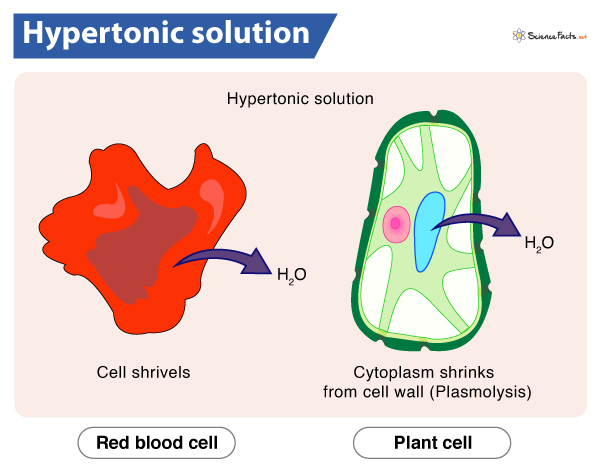
As hypertonic solution contains more solutes and less water than the cell sap, the cell will lose water and eventually shrink.
Following the rule of osmosis, water will start to move from its region of higher concentration to lower across the cell membrane, i.e., from the cell to the external medium (exosmosis). Thus a cell maintains the equilibrium of water content both internally and externally.
Examples of Hypertonic Solution
In Animals
Humans contain specialized proteins called osmoreceptors in their brains, which regulate the water content in their bodies. These proteins measure the osmolarity (total number of solute particles per liter) of their surrounding environment and act accordingly.
For instance, if the external medium of kidney cells turns hypertonic, the hypothalamus releases Vasopressin (ADH, antidiuretic hormone). It increases the water permeability of the renal collecting duct cells, allowing more water to get reabsorbed from the urine of the collecting duct to blood.
As seawater is hypertonic, it tends to pull out water from the cells of aquatic organisms living there. So, marine animals, such as sea turtles, need to maintain a more hypertonic internal environment to survive. As a result, they prevent excessive water loss and remove excess salt through the salt gland to maintain osmotic balance.
In Plants
In plants, if the external medium becomes hypertonic, water moves out of the cell following its concentration gradient. As a result, the cell cytoplasm gets shrunk and detaches itself from the cell membrane. This phenomenon is known as plasmolysis, and the cell is called plasmolyzed or flaccid. Overall, the plant appears wilted.
That is why plants usually prefer a hypotonic environment over a hypertonic one.
As in hypotonic surroundings, water enters into the cell, allowing plants to maintain structural rigidity.
At Cellular Level
Red Blood Cells (RBCs) form irregular notched surfaces (crenation) due to excessive water loss upon exposure to a hypertonic solution.
Hypertonic vs. Hypotonic Solution
Let us now discuss the fundamental differences between these two solutions:
| Hypertonic Solution | Hypotonic Solution |
|---|---|
| Has a higher solute concentration than the cell interior or cell sap. | Has a lower solute concentration than the cell interior or cell sap. |
| Causes exosmosis, i.e., water moves out of the cell. | Causes endosmosis, i.e., water moves into the cell. |
| Cell shrinks and loses its shape. | Cell swells up and eventually bursts. |
| Higher osmotic pressure than cell interior. | Lower osmotic pressure than cell interior. |
| Example: Sugar syrup is hypertonic solution for a cell. | Example: Freshwater is hypotonic for saltwater fish. |
FAQs
Ans. To determine if a solution is hypertonic or hypotonic, we need to place a cell in it. If the cell swells up, it means there is an inward movement of water, referring to the solution being hypotonic. On the other hand, if the cell shrinks due to the outward movement of water, it can be concluded that the solution is hypertonic.
-
References
Article was last reviewed on Friday, December 17, 2021